Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Osteoarthritis (OA) is a serious disease characterized by progressive joint failure and cartilage degeneration. In OA, ADAMTS-5 protease is upregulated, resulting in enhanced cleavage of joint cartilage aggrecan into fragments (e.g. the aggrecan neo-epitope family [ARGS]) and subsequent stimulation of synovial inflammation. M6495 is an anti-catabolic, bivalent, bifunctional Nanobody® that selectively inhibits ADAMTS-5 in vitro, reducing aggrecan cleavage. This Phase I, single-center, randomized, double-blind, placebo-controlled, single ascending dose (SAD), first-in-human (FIH) study (NCT03224702) was conducted in healthy male subjects to explore the safety, tolerability, immunogenicity, pharmacokinetics (PK), and pharmacodynamics (PD) of single subcutaneous doses of M6495, with the aim to guide clinical development.
Methods: Male subjects (18–55 years old, body mass index of ≥18.5–≤29.9 kg/m2) were randomized 2:1 to either M6495 or placebo in 6 dose-level (DL) cohorts (1 mg, 5 mg, 20 mg, 75 mg, 150 mg, 300 mg). Primary endpoints were safety and tolerability. Secondary endpoints were serum-based M6495 PK parameters and PD parameters, including percent change from baseline in ARGS. Exploratory endpoints included the effects of M6495 on versican degradation fragment (VCANM) and high-sensitivity C-reactive protein (hsCRP).
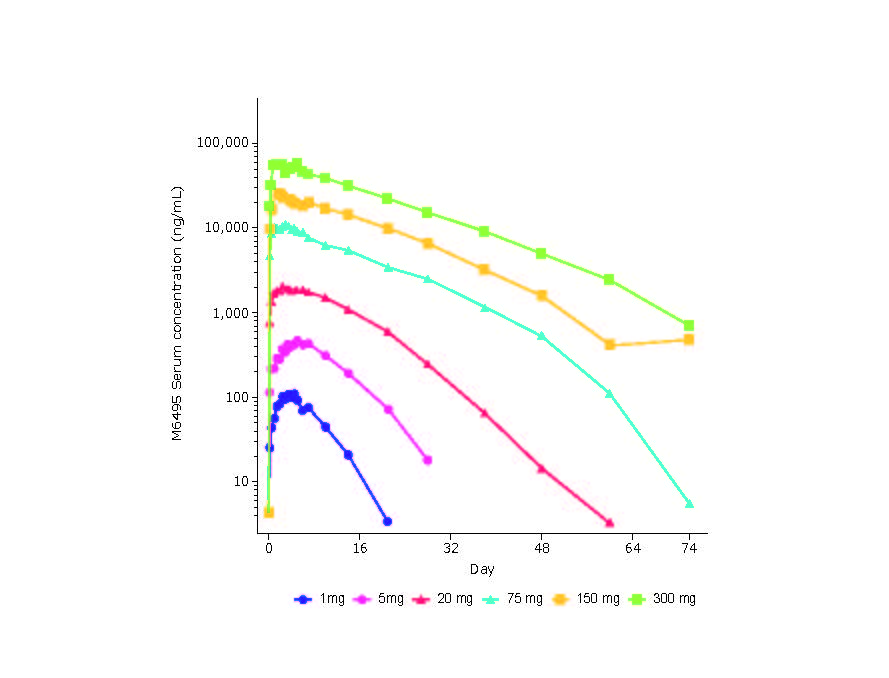
Results: 53/54 randomized healthy subjects completed the study; 1 discontinued due to an internship in Africa. Overall, there was minimal difference in treatment-emergent adverse event (TEAE) rate either between each M6495 DL cohort or between M6495 and placebo; 28 (77.8%) subjects receiving M6495 and 12 (66.7%) receiving placebo experienced TEAEs. The most common TEAEs are shown in Table 1; the majority were of mild intensity. Observed injection site reactions were mainly mild in nature and within expectations for a therapeutic protein. No deaths or safety signals emerged based on reported TEAEs or laboratory measurements. Based on serum AUC and Cmax, M6495 exposure increased in a slightly greater-than-dose-proportional manner across the dose range (Figure 1). Median M6495 tmax ranged from 24–120 hours following administration of M6495. ARGS levels were reduced from baseline in a dose-dependent manner (Figure 2). Overall, 6 subjects had anti-drug antibodies (ADA); 2 in the placebo cohort and 1 each in the 5 mg, 20 mg, 75 mg and 300 mg cohorts. In 1 subject, ADA occurred at Day -1. Presence of ADA did not affect PK. In the 300 mg cohort, ARGS levels decreased by up to 78% vs baseline (95% CI = [54, 100]) and up to 93% vs placebo (mean [95% CI]: 93% [65, 120]) by electrochemiluminescence immunoassay. Following administration of 300 mg M6495, an ~45% decrease in mean percentage change from baseline serum ARGS was maintained through 74 days (last planned time point) vs placebo, whereas reductions of >30% were maintained up to 60 days in the 75 mg and 150 mg cohorts. No significant modulation of hsCRP or VCANM were detected.
Conclusion: The observed safety profile of M6495 at single doses up to 300 mg appeared to be acceptable for further clinical development. M6495 dose-dependently inhibited release of the ARGS aggrecan fragment family.
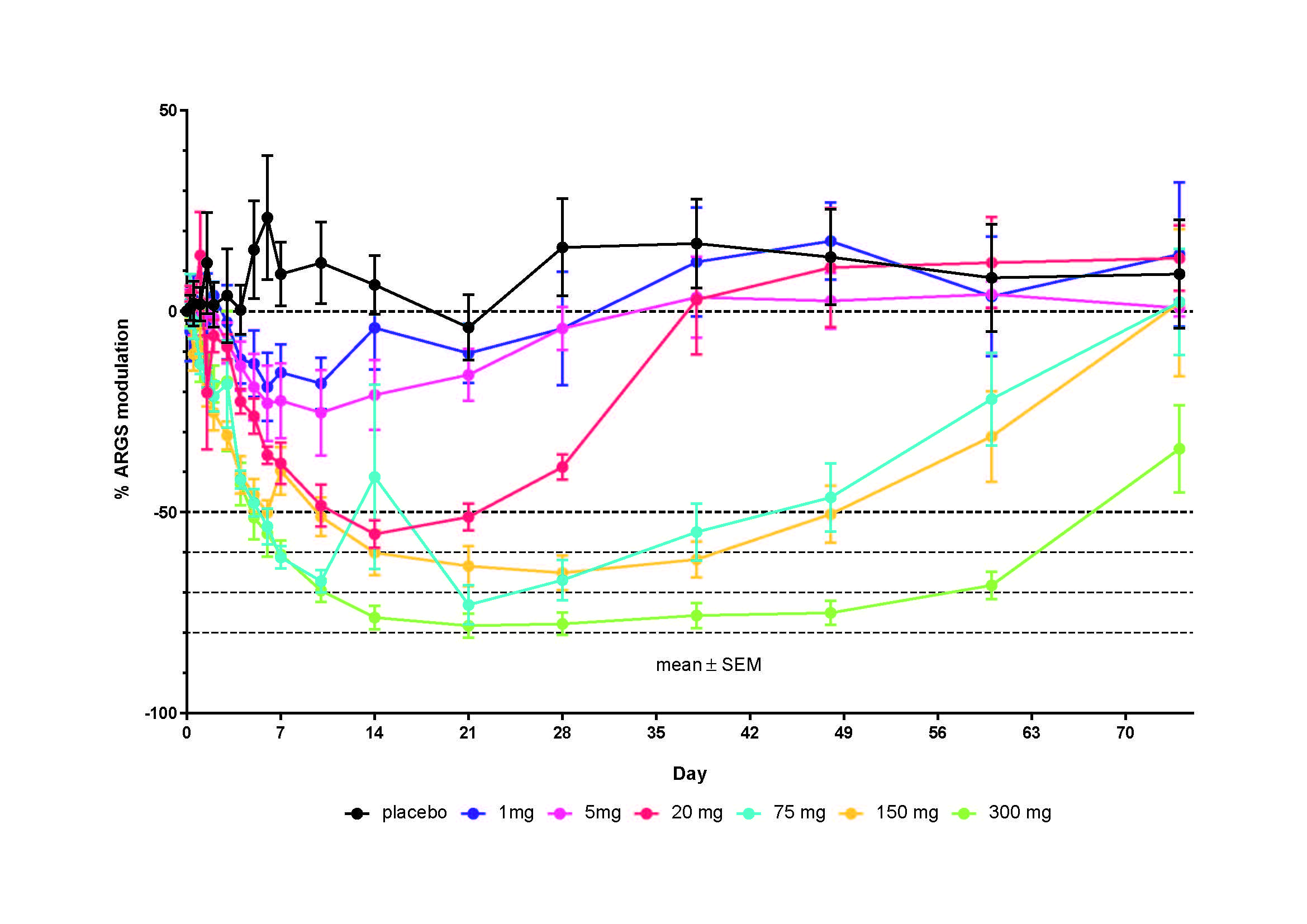
20050 M6495 Figure 1 V2 -24 MAY 19-
20050 M6495 Figure 2 V4 -24 MAY 19-
To cite this abstract in AMA style:
Guehring H, Balchen T, Goteti K, Sonne J, Ladel C, Ona V, Moreau F, Bay-Jensen A, Reinstrup Bihlet A. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single Ascending Doses of the Anti-ADAMTS-5 Nanobody®, M6495, in Healthy Male Subjects: A Phase I, Placebo-Controlled, First-in-Human Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-single-ascending-doses-of-the-anti-adamts-5-nanobody-m6495-in-healthy-male-subjects-a-phase-i-placebo-controlled-first-in-huma/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-single-ascending-doses-of-the-anti-adamts-5-nanobody-m6495-in-healthy-male-subjects-a-phase-i-placebo-controlled-first-in-huma/

