Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The purpose of this study was to evaluate the safety of bone-marrow derived allogeneic mesenchymal stem cells (MSCs) as therapeutic agents in early rheumatoid arthritis (RA). Used to reduced graft-versus-host disease following bone marrow transplantation, MSCs are being clinically tested in multiple sclerosis, Crohn’s disease, cystic fibrosis and other diseases for their immune regulatory properties. We designed and conducted a Phase 1 randomized double blind clinical trial (RDBCT) using MSCs in early RA. MSCs have immunomodulatory properties ex vivo and in vivo. We hypothesized that a “window of opportunity” would exist in early RA during which immune tolerance could be restored to a “pre-RA-like” state
Methods: We conducted a Phase 1 RDBCT in early RA patients using a single infusion of marrow derived allogeneic MSCs. Donors were pre-screened and MSCs isolated and expanded and tested in functional suppressor assays ex vivo. The selected MSC donor had a second bone marrow aspiration and MSCs were expanded in the Case Comprehensive Cancer Center GMP faculty and cryopreserved. MSCs underwent purity and adventitious testing in advance and endotoxin prior to release. Studies were perfumed under IND # 016906 and according to the Declaration of Helsinki. Patients enrolled had symptoms of RA for less than two years and had elevated rheumatoid factor or antibody directed against citrullinated peptides or both. Known DMSO sensitivity, active infection and history of disease modifying drugs (DMARDs) other than methotrexate, hydroxychloroquine and low dose prednisone were exclusionary.
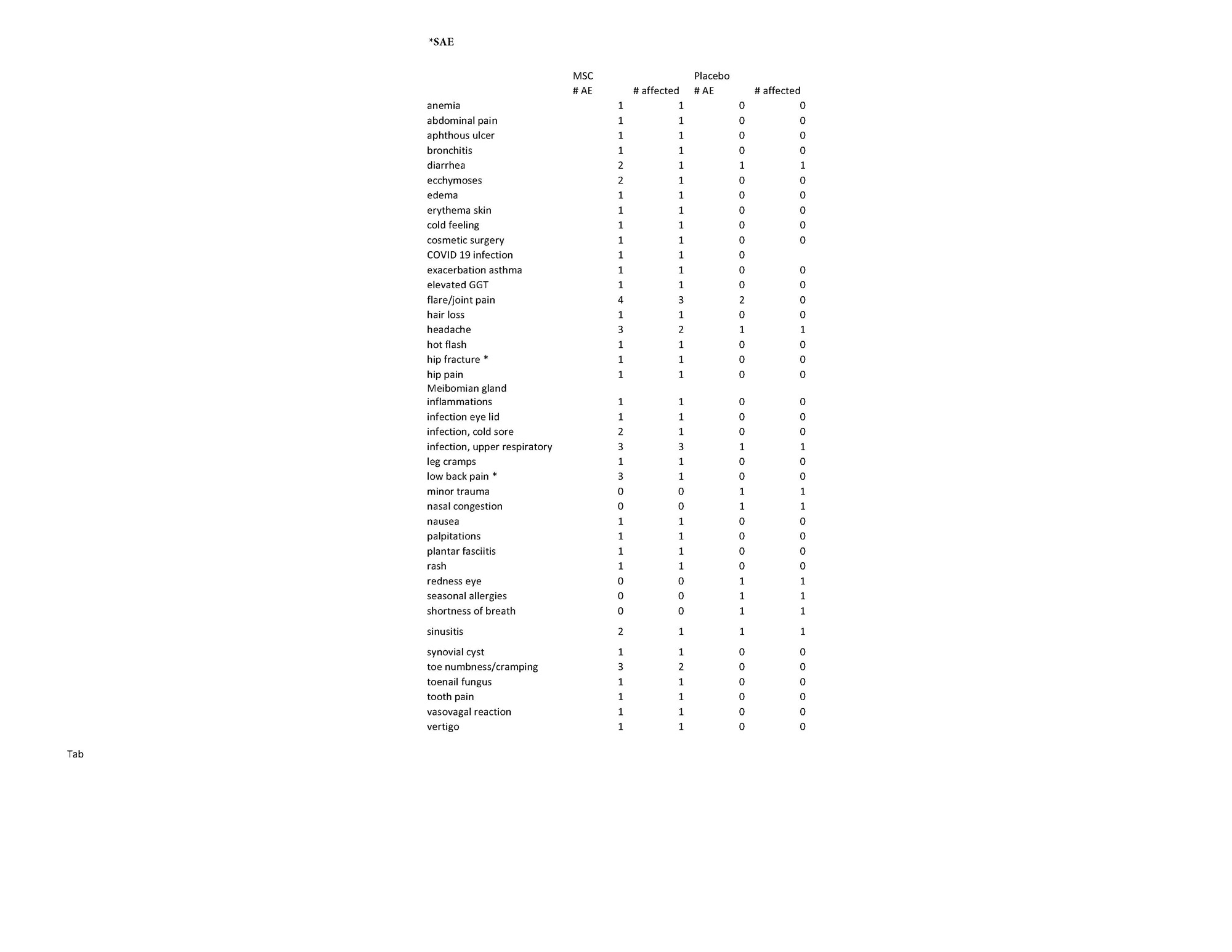
Results: Screening lasted 42 days prior to infusion. Of the ten patients treated, five were administered MSCs at 2 million (M)/kg and three patients at 4M/kg and two received sham infusion. Patients were pre-medicated with acetaminophen and diphenhydramine. There were no dose limiting toxicity and no infusion reactions. Two SAEs were not considered to be study related: 1) acute exacerbation of chronic low back pain; 2) hip fracture in a patient on low dose steroids. Enrollment was halted after ten patients due to stalling during the pandemic. Demographics are included in Table 1. Spirometry post-infusion was discontinued with permission mid-way through the trial as no respiratory AEs occurred in conjunction with or immediately after infusion. No study related AEs related to the therapeutic agent were reported (Table 2). Secondary outcomes including Patient Reported Outcomes and DAS28CRP are under analysis.
Conclusion: Use of “off the shelf” bone marrow derived adult “mesenchymal stem cells” appear safe using dosages up to 4M/Kg. There were no dose limiting toxicities observed and patients tolerated infusion well without pre-conditioning, as is required for use of other cellular therapies. Given the safety of using MSC’s, we suggest that allogeneic MSCs should be tested early in RA in a phase 2 study for their ability to induce immune quiescence and induction of host regulatory cells as well as efficacy responses.
To cite this abstract in AMA style:
Singer N, Breitman M, Haghiac M, Gordesky L, Reese J, Lewis S, Barnboym E, Hussein N, Lasalvia S, Mellins E, Bonfield T, Anthony D, Caplan A, Lazarus H. Safety Results from a Phase 1 Double-blind Randomized Clinical Trial of Allogeneic Mesenchymal Stem Cells in Early RA [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-results-from-a-phase-1-double-blind-randomized-clinical-trial-of-allogeneic-mesenchymal-stem-cells-in-early-ra/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-results-from-a-phase-1-double-blind-randomized-clinical-trial-of-allogeneic-mesenchymal-stem-cells-in-early-ra/