Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA) has shown efficacy and safety in patients (pts) with active PsA in the Phase 3 SELECT-PsA 1 and SELECT-PsA 2 clinical trials. 1,2 Here we present the integrated safety data from the placebo (PBO)-controlled 24-week period of the clinical program.
Methods: The SELECT-PsA program enrolled pts with prior inadequate response (IR) or intolerance to ≥1 non-biologic DMARD (non-bDMARD) and prior IR or intolerance to ≥1 bDMARD. Both trials evaluated PBO, UPA 15 mg once daily (QD; UPA15) and UPA 30 mg QD (UPA30); SELECT-PsA 1 also included the active comparator adalimumab (ADA).1,2 Data was integrated for pts randomized to PBO, UPA15, or UPA30. Pts were permitted to be on monotherapy or on up to 2 non-biologic DMARDs concomitantly. Treatment-emergent adverse events (TEAEs) from the trials were analyzed and summarized. The number and percentage of pts with TEAEs through Week 24 are presented.
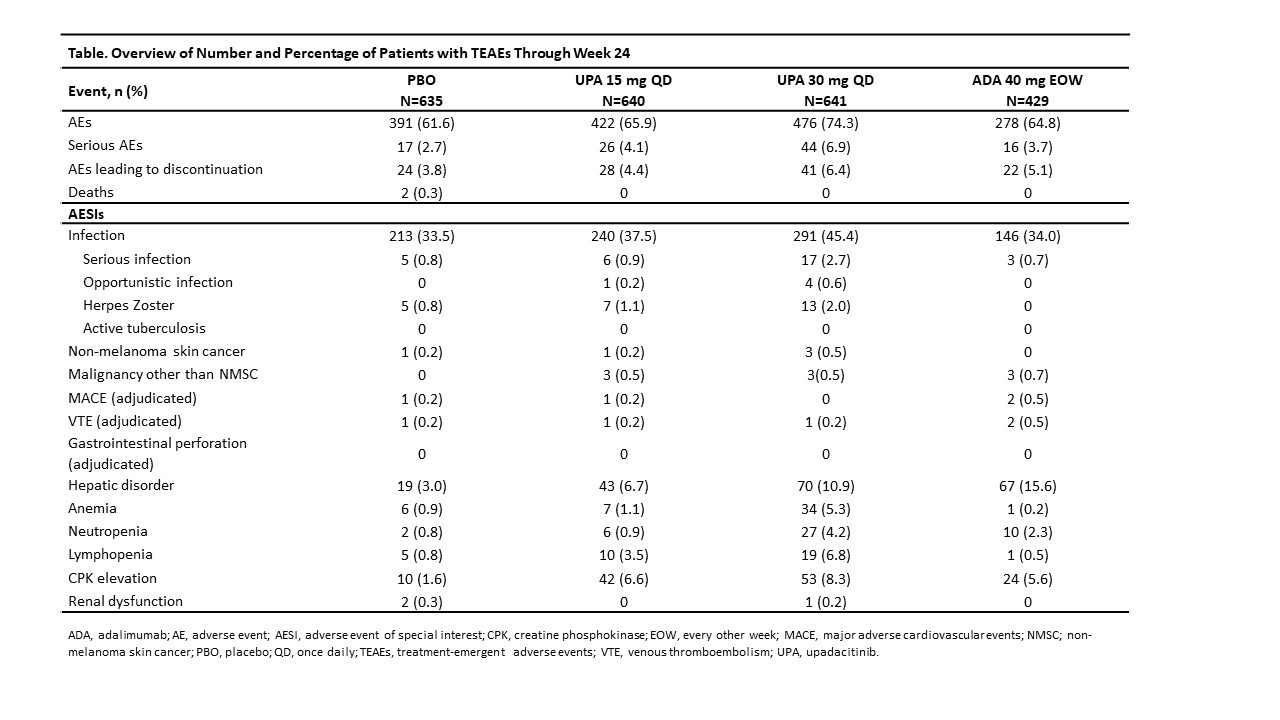
Results: The number of pts who received ≥ 1 dose of study drug and AEs are presented in the Table. The rates of TEAEs, serious AEs, and AEs leading to study drug discontinuation (D/C) were similar with PBO and UPA15 and higher with UPA30. Rates of serious infection (SIs) and herpes zoster (HZ) were similar with PBO and UPA15 and higher with UPA30. The most common SI was pneumonia. Most HZ events involved one dermatome; no central nervous system infection was reported. Opportunistic infections included 1 event of candida urethritis with UPA15, and 1 event each of pneumocystis jirovecii pneumonia, cytomegalovirus, candidiasis of the trachea, and oropharyngeal candidiasis with UPA30. Malignancies other than NMSC were reported at similar rates with UPA15 and UPA30; no events were reported with PBO. The rate of NMSC was similar with PBO, UPA15, and UPA30. The number of adjudicated major adverse cardiovascular events (MACE) and venous thromboembolic events (VTE) was < 0.5% for all arms, with similar rates reported with PBO, UPA15, and UPA30; all pts had at least one risk factor. VTEs included 1 deep vein thrombosis in PBO and 1 pulmonary embolism each in the two UPA arms. There were no adjudicated gastrointestinal perforations. Hepatic disorders were mostly transient transaminase increases. CPK elevations were reported more frequently in the UPA arms vs PBO and were mostly asymptomatic, and rarely led to study drug D/C; no rhabdomyolysis was reported. AEs of anemia, neutropenia, and lymphopenia were generally mild or moderate, non-serious, and uncommonly led to study drug D/C. The safety profile was generally consistent between UPA15 and ADA, except for lower rates of HZ, anemia, and lymphopenia and higher rates of hepatic disorders and neutropenia in the ADA arm. In contrast, the rates of most AEs were higher with UPA30 compared to ADA except for hepatic disorders.
Conclusion: The safety profiles of UPA15 and ADA were generally consistent; the rates of most AEs were higher with UPA30 compared to ADA. Through Week 24, the safety profile of UPA15 and UPA30 in PsA pts demonstrated consistent results compared to what has been observed with UPA in RA pts.3
References
1. McInnes IB, et al. Ann Rheum Dis, 2020; 79:12.
2. Genovese MC, et al. Ann Rheum Dis, 2020; 79:139.
3. Cohen SB, et al. Thu0167. Annals of the Rheumatic Diseases. 2019; 78:357
To cite this abstract in AMA style:
Burmester G, Winthrop K, Nash P, Goupille P, Azevedo V, Salvarani C, McCaskill R, Liu J, Pierre-Louis B, Anderson J, Ruderman E. Safety Profile of Upadacitinib in Psoriatic Arthritis: Integrated Analysis from Two Phase 3 Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/safety-profile-of-upadacitinib-in-psoriatic-arthritis-integrated-analysis-from-two-phase-3-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-profile-of-upadacitinib-in-psoriatic-arthritis-integrated-analysis-from-two-phase-3-trials/