Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Enpatoran, a highly selective, potent and reversible dual toll-like receptor 7/8 (TLR7/8) inhibitor, targets key innate and adaptive immune processes involved in the pathogenesis and progression of SLE and CLE, and has glucocorticoid-sparing potential. Enpatoran was well tolerated with no safety signals in all clinical trials to date, including a phase 2 study in COVID-19 pneumonia. The objective of this study was to evaluate the safety, pharmacokinetics (PK), pharmacodynamics and clinical efficacy of multiple-ascending doses of enpatoran in patients with active SLE and CLE.
Methods: This phase 1b study (NCT04647708) of oral enpatoran was conducted in patients with active SLE (≥1 clinical manifestation by Systemic Lupus Erythematosus Disease Activity Index 2000 [SLEDAI-2K] and/or Cutaneous Lupus Erythematosus Disease Area and Severity Index-A [CLASI-A] ≥6), or active CLE (diagnosis of SCLE and/or DLE; CLASI-A ≥6). All patients with SLE fulfilled ≥4 ACR classification criteria. There were 3 dose-escalating cohorts (25, 50, 100 mg twice daily [BID] vs placebo) and 1 optional cohort (150 mg BID vs placebo). In each cohort, patients were randomized 3:1 to enpatoran or placebo. The 12-week treatment period was extended to 24 weeks for the 100 mg and 150 mg BID cohorts. All cohorts had a 2-week safety follow-up period. The primary endpoint was safety and tolerability. Secondary endpoints included PK and change in SLEDAI-2K scores. Change in IFN-gene signature (IFN-GS) scores was an exploratory endpoint.
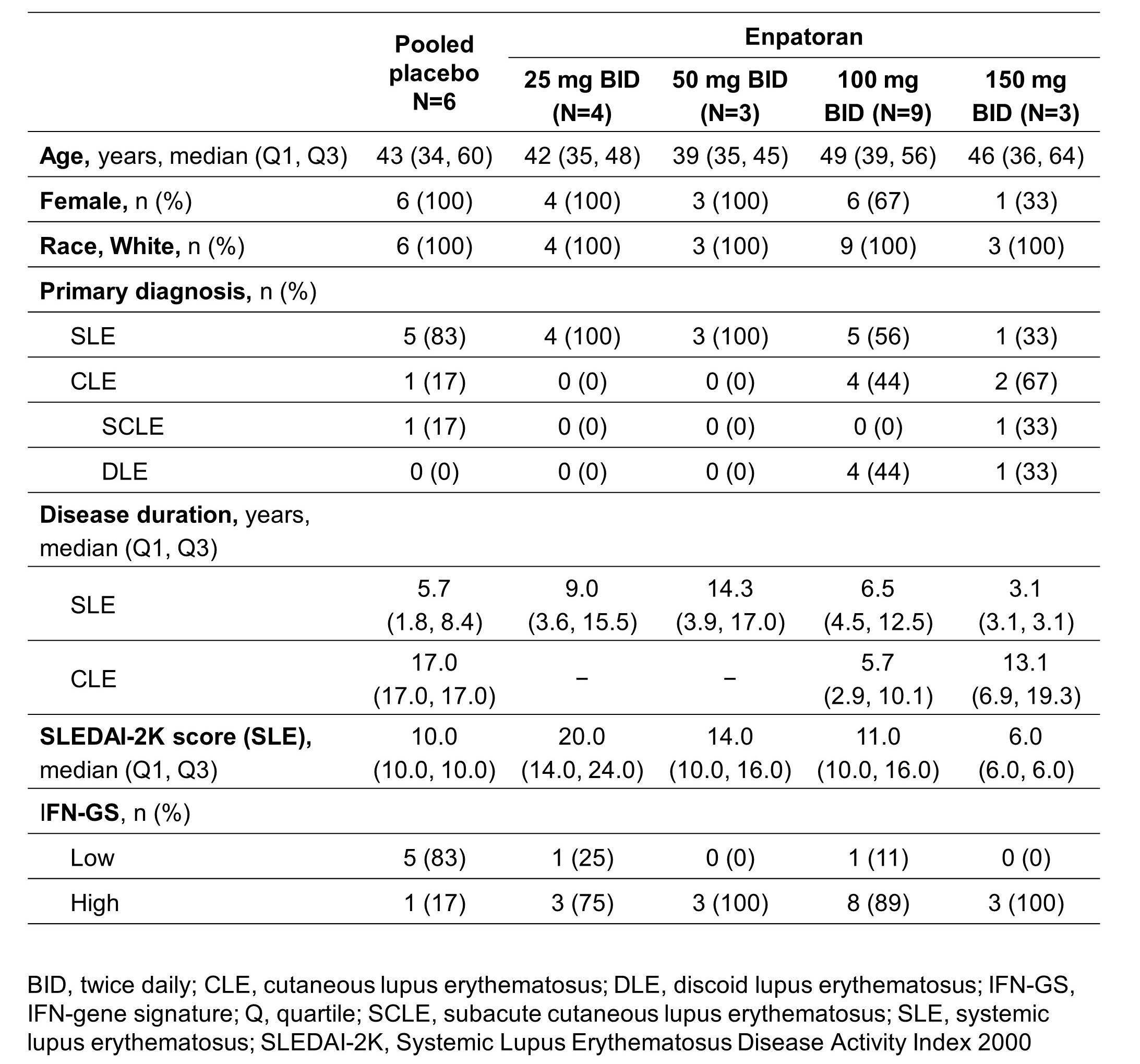
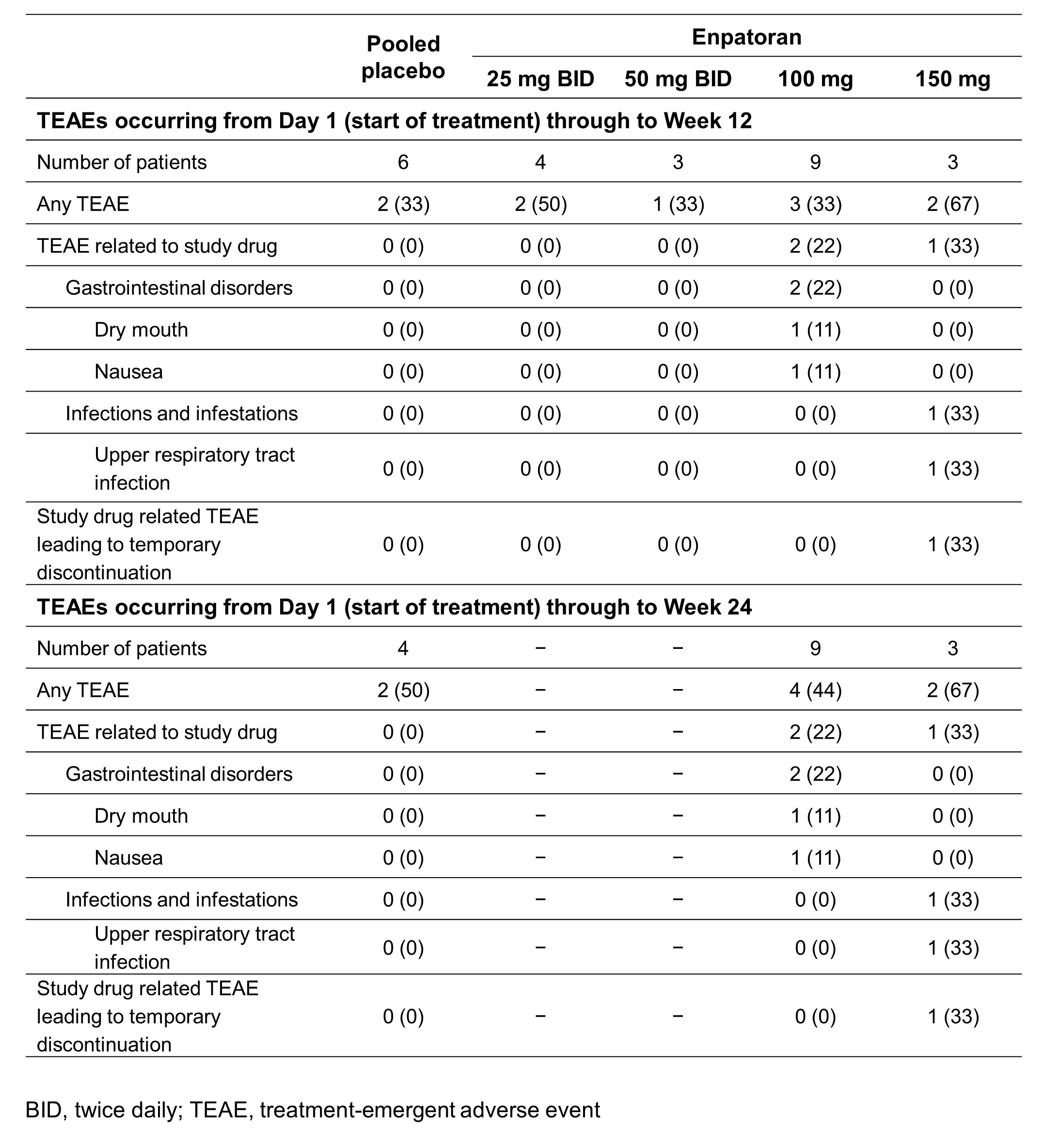
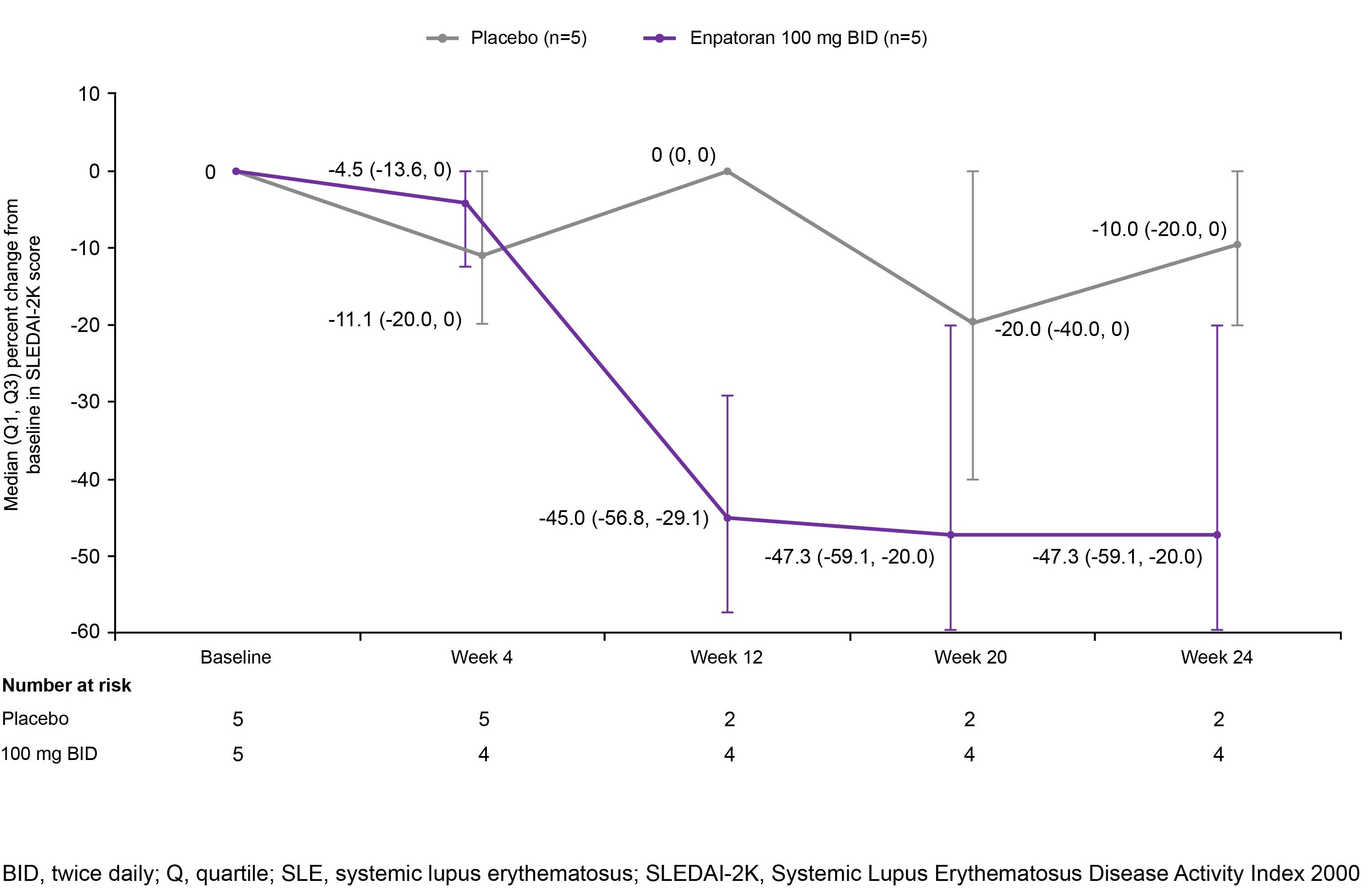
Results: 25 patients were randomized to enpatoran or placebo in the 25 mg (n=5), 50 mg (n=4), 100 mg (n=12) and 150 mg (n=4) BID cohorts (SLE: n=18; CLE: n=7). Patient characteristics are shown in Table 1. Enpatoran was well tolerated, with 8 patients (42%) experiencing ≥1 treatment-emergent adverse event (TEAE) by Week 12 (Table 2). There were no deaths, serious TEAEs, AEs of special interest or dose-limiting toxicities. One patient in the 25 mg BID group experienced a treatment-unrelated Grade 4 TEAE of worsening lymphopenia. One TEAE considered related to enpatoran (150 mg BID; upper respiratory tract infection) resulted in a temporary discontinuation in that patient. Enpatoran remained well tolerated to Week 24 with 100 mg and 150 mg BID (Table 2). PK profiles were consistent and dose proportional across enpatoran doses, with a half-life of 6‒10 hours. In SLE patients, numerically greater reductions in SLEDAI-2K scores were observed with enpatoran 100 mg BID versus placebo after Week 4 and were maintained to Week 24 (Figure 1). Enpatoran reduced IFN-GS scores in a dose-dependent manner.
Conclusion: Enpatoran was well tolerated up to the highest evaluated dose of 150 mg BID for 24 weeks and demonstrated favorable safety and PK profiles in the first clinical study in SLE and CLE. The numerical reduction in SLEDAI-2K scores with enpatoran 100 mg BID and suppression of IFN-GS suggest a pharmacological effect. Enpatoran is being evaluated in a larger cohort of patients with SLE and/or CLE in an ongoing Phase II study (WILLOW; NCT05162586).
Medical writing support was provided by Bioscript Group.
To cite this abstract in AMA style:
Witte T, Fernandez Ruiz R, Abramova N, Weinelt D, Moreau F, Klopp-Schulze L, Shaw J, Denis D, Wenzel J. Safety, Pharmacokinetics, Clinical Efficacy and Exploratory Biomarker Results from a Randomized, Double-Blind, Placebo-Controlled Phase 1b Study of Enpatoran in Active Systemic and Cutaneous Lupus Erythematosus (SLE/CLE) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-pharmacokinetics-clinical-efficacy-and-exploratory-biomarker-results-from-a-randomized-double-blind-placebo-controlled-phase-1b-study-of-enpatoran-in-active-systemic-and-cutaneous-lupus-ery/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-pharmacokinetics-clinical-efficacy-and-exploratory-biomarker-results-from-a-randomized-double-blind-placebo-controlled-phase-1b-study-of-enpatoran-in-active-systemic-and-cutaneous-lupus-ery/