Session Information
Session Type: Poster Session B
Session Time: 5:00PM-6:00PM
Background/Purpose: The management of pediatric rheumatic disease has been forever changed by the advent of biologic drugs and the pursuit of targeted therapy. There is growing literature on the safety and efficacy of combining synthetic and biologic disease modifying anti-rheumatic drugs (DMARDs). However, in rare instances, patients continue to have breakthrough disease activity despite escalation of conventional therapies, and rheumatologists must consider the synergistic use of combined biologics. Currently, there is a paucity of literature describing the use of combined biologics in pediatrics. We aim to describe the short-term safety outcomes of a cohort of pediatric rheumatology patients treated with a combined biologics regimen (CBR).
Methods: In this single-center retrospective chart review, we identified pediatric patients treated with combined biologics for a systemic autoimmune disease between 2020-2022 at Texas Childrens Hospital. With IRB approval, we extracted clinical features, immunomodulation use, and outcomes for these patients.
Results: Eight patients were identified: 6 with systemic Juvenile Idiopathic Arthritis (JIA) and 2 with seronegative polyarticular JIA. Median age at time of CBR initiation was 6.5 years (range 3-18). All patients had severe, refractory disease, and all had failed multiple synthetic and biologic DMARDs prior to CBR initiation. Two patients were trialed on more than one CBR.
Table 1 summarizes CBR-related outcomes. While on CBR, two patients were hospitalized for infection (viral gastroenteritis and cutaneous herpes zoster respectively), and CBR was subsequently discontinued for one. One patient was hospitalized for pneumothoraces associated with implanted port access. Two patients are monitored for mild transaminitis attributed to pharmacotherapy.
Though 6 patients were able to come off of chronic oral steroids after CBR initiation, none of the patients were able to maintain clinical and serologic remission for longer than 6 months.
Conclusion: Majority of patients did not develop serious infections or adverse events requiring hospitalization while on CBR. Additional prospective cohort studies are needed to determine the efficacy and long-term outcomes for pediatric patients on combined biologics.
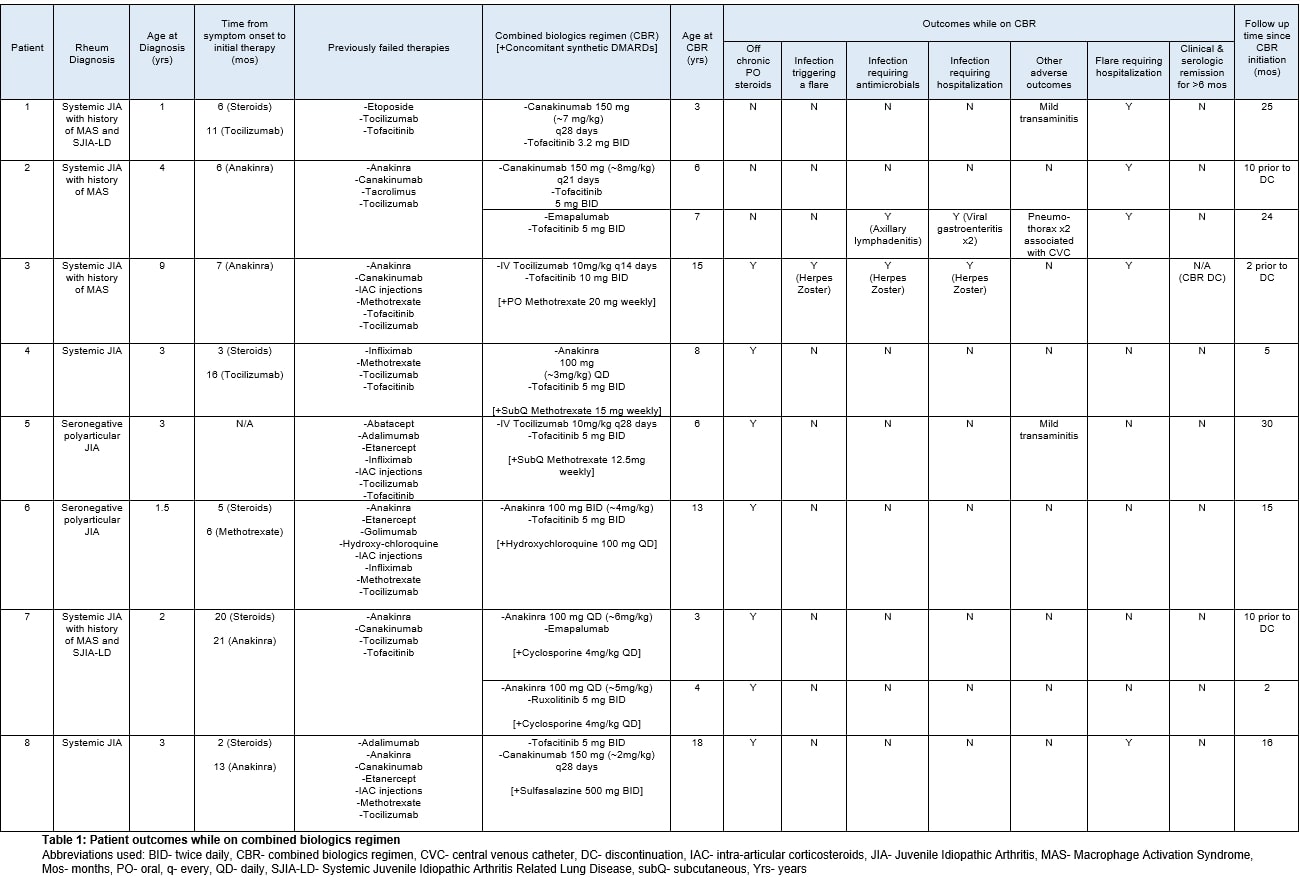 Table 1: Patient outcomes while on combined biologics regimen
Table 1: Patient outcomes while on combined biologics regimen
Abbreviations used: BID- twice daily, CBR- combined biologics regimen, CVC- central venous catheter, DC- discontinuation, IAC- intra-articular corticosteroids, JIA- Juvenile Idiopathic Arthritis, MAS- Macrophage Activation Syndrome, Mos- months, PO- oral, q- every, QD- daily, SJIA-LD- Systemic Juvenile Idiopathic Arthritis Related Lung Disease, subQ- subcutaneous, Yrs- years
To cite this abstract in AMA style:
Chun A, Nguyen M, De Guzman M, Ramirez A. Safety Outcomes of Combined Biologics Use in Pediatric Rheumatology: A Single Center Experience [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/safety-outcomes-of-combined-biologics-use-in-pediatric-rheumatology-a-single-center-experience/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-outcomes-of-combined-biologics-use-in-pediatric-rheumatology-a-single-center-experience/
