Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: To assess the most recent safety-data of synthetic (s) and biological (b)DMARDs to inform the 2019 update of the EULAR recommendations for the management of RA.
Methods: Observational studies comparing any DMARD with another intervention for the management of patients with RA were identified by searches in MEDLINE, Embase and Cochrane (time-window: 2016-2019). Interventions included any bDMARD, csDMARD, tsDMARD or glucocorticoids. A comparator group was required for the study to be included. All safety outcomes were included. Observational studies were chosen because they better reflect routine clinical care. For treatments still without registry data, randomized controlled trials (RCTs) and long-term extensions (LTEs) thereof were used to extract safety data. Risk of bias was assessed according to the Hayden’s tool for observational studies and Cochrane Collaboration’s tool for RCTs.
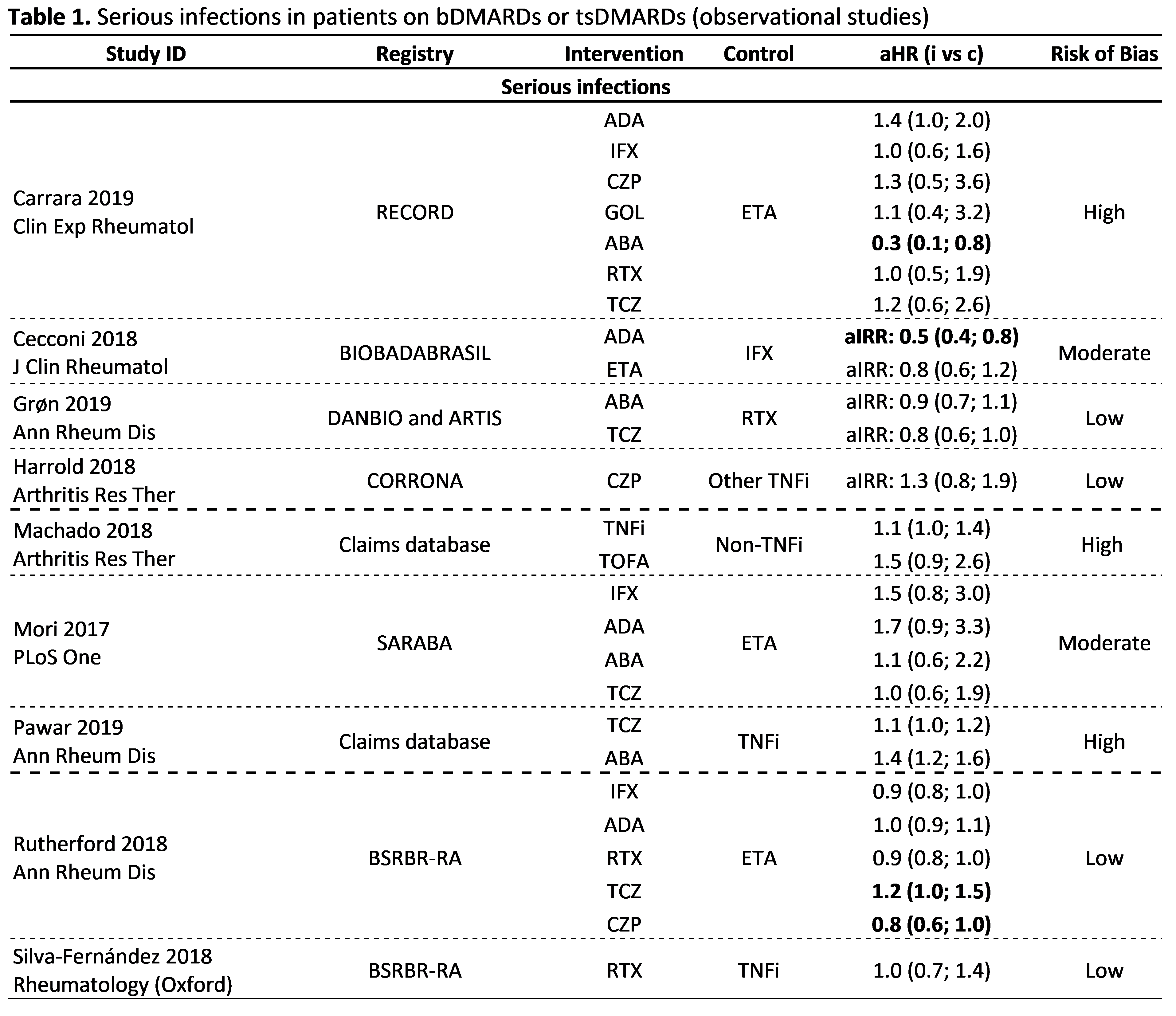
Results: Of 3,886 articles screened, 42 observational studies fulfilled the inclusion criteria, with 16 focusing on the risk of infections, 8 on cancer, 10 on major cardiovascular events (MACE), 3 on lower intestinal perforations (LIP), 5 on withdrawals due to adverse events and 2 on immunological reactions. Studies were heterogeneous precluding meta-analysis. Two studies showed an increased risk of serious infections (SI) with bDMARDs compared to csDMARDs (aIRR: 3.1-3.9). In 9 studies, 4 at low risk of bias, the risk of SI was not different between bDMARDs, with one also showing no difference between tofacitinib and TNFi (Table 1). Two studies (high risk of bias) showed no difference in the risk of infection by herpes zoster across bDMARDs, but in one, an increased risk for tofacitinib compared to abatacept was reported. Five studies (4 at low risk of bias) showed no increased risk of cancer for bDMARDs compared to csDMARDs; and 2 (at low risk of bias) no difference between bDMARDs. The risk of MACE did not differ between bDMARDs and csDMARD (3 studies, 1 at low risk of bias) and also comparing different bDMARDs (4 studies, 1 at low risk of bias). An increased risk of LIP was found for tocilizumab compared to csDMARDs (1 study, low risk of bias) and to TNFi (2 studies, high risk of bias). In total, 57 manuscripts reported safety data from RCTs/LTEs, 21 evaluating biosimilars, 18 bDMARDs and 18 tsDMARDs. Overall, no unexpected safety outcomes were found, except for the possible increased risk of venous thromboembolism (VTE) with tsDMARDs (Table 2). This signal prompted FDA/EMA to issue warnings to avoid baricitinib 4mg (not 2mg) and tofacitinib 10 mg bid (not 5mg bid) in patients with risk factors for VTE.
Conclusion: Data obtained by this SLR are aligned with previous evidence; they show a slightly increased risk of SI with bDMARDs compared to csDMARDs and no difference between bDMARDs. The overall risk of cancer and MACE was not increased with bDMARDs compared with csDMARDs. The risk of LIP with tocilizumab and VTE with tsDMARDs needs further evaluation.
To cite this abstract in AMA style:
Sepriano A, Kerschbaumer A, Smolen J, van der Heijde D, Dougados M, Van Vollenhoven R, McInnes I, Bijlsma J, Burmester G, de Wit M, Falzon L, Landewé R. Safety of Synthetic and Biological DMARDs: A Systematic Literature Review Informing the 2019 Update of the EULAR Recommendations for Management of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-of-synthetic-and-biological-dmards-a-systematic-literature-review-informing-the-2019-update-of-the-eular-recommendations-for-management-of-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-synthetic-and-biological-dmards-a-systematic-literature-review-informing-the-2019-update-of-the-eular-recommendations-for-management-of-rheumatoid-arthritis/