Session Information
Date: Sunday, November 8, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The
aim of this study was to investigate the frequency and risk factors of
postoperative complications in rheumatoid arthritis (RA) patients treated with abatacept
in routine care.
Methods: The
ORA registry is a French nationwide prospective cohort which recruited 1032
patients receiving abatacept for RA according to ACR criteria in routine care.
Data for patients who underwent surgery while treated with abatacept (including
the 4 months following last infusion) were reviewed to describe the frequency
of postoperative complications. Characteristics of patients and surgeries were
compared between patients with and without complications to identify factors
associated with complications. Quantitative variables were compared by the
Mann-Whitney test, and qualitative variables were compared by the Fisher’s
exact test.
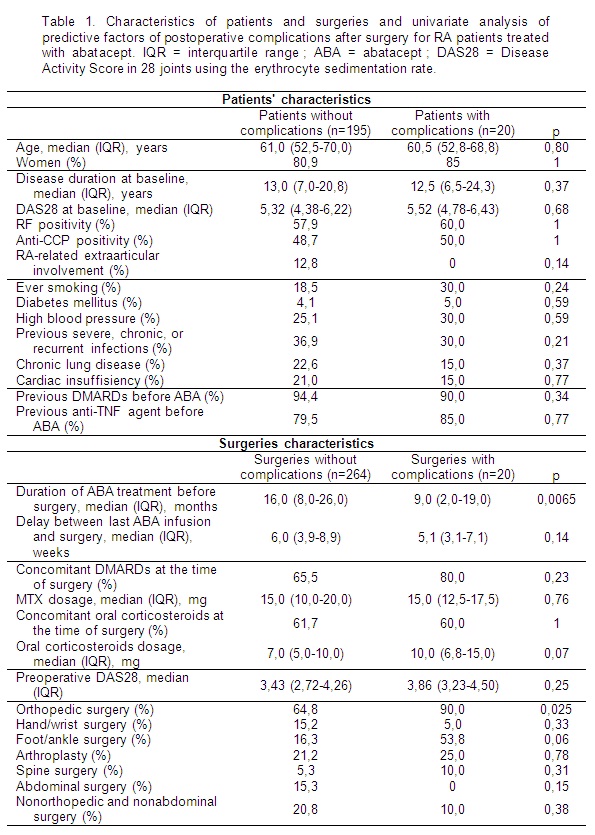
Results: We
identified 215 (20.8 %) patients who underwent 284 surgeries between June 2008
and December 2012, including 189 (66.5%) orthopedic surgeries and 35 (12.3%)
abdominal surgeries. Twenty (7.0%) surgeries, in 20 patients (9.3%), entailed
complications, of which 11 infections (3.9% of surgeries) and 6 delayed healing
(2.1% of surgeries). Abatacept was discontinued after 3 (15.0%) surgeries in
the complicated group versus 28 (10.6%) in the group without complications, and
this difference was not significant (p=0.467). No death was reported. Characteristics
of patients and surgeries with and without complications are shown in table 1. Co-morbidities
and associated treatments were not different between both groups. The median time
between the last infusion of abatacept and surgery was 5.9 weeks (interquartile
range [IQR] 3.8-8.8 weeks), with no difference between patients with and
without complications (5.1 vs 6.0 weeks, p=0.14). The median time between the initiation
of abatacept and surgery was significantly shorter in the complicated group (9
[IQR 2-19] vs. 16 months [IQR 8-26], p=0.0065), while DAS28 score at the time
of surgery was similar between both groups (3.86 vs 3.43, p=0,25). Orthopedic
surgeries were associated with a higher rate of postoperative complications
(9,5% vs. 2,1%, p=0,025), but no difference was found in each subgroup of
orthopedic procedures (i.e. hand/wrist, foot/ankle, spine surgeries or
arthroplasty).
Conclusion: In real
life RA patients treated with abatacept and undergoing surgery, there is no
specific predictive factor of complications except an orthopedic vs
non-orthopedic procedure (like in every RA patient) and a shorter time after
initiation of abatacept. The latter factor, also found in our rituximab AIR registry
(Godot et al, Arthritis Care Research 2013), is probably a bias due to the
healthy drug survival effect. Interestingly, the median time between the last
infusion of abatacept and the surgery was short: 5 to 6 weeks and did not
influence the rate of post-operative complications.
To cite this abstract in AMA style:
Latourte A, Gottenberg J, Luxembourger C, Pane I, Claudepierre P, Richette P, Lafforgue P, Ravaud P, Combe B, Cantagrel AG, Sibilia J, Flipo RM, Gaudin P, Vittecoq O, Schaeverbeke T, Dougados M, Berenbaum F, Sellam J, Mariette X, Seror R. Safety of Surgery in Patients with Rheumatoid Arthritis Treated By Abatacept: Data from the French Orencia in Rheumatoid Arthritis (ORA) Registry [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/safety-of-surgery-in-patients-with-rheumatoid-arthritis-treated-by-abatacept-data-from-the-french-orencia-in-rheumatoid-arthritis-ora-registry/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-surgery-in-patients-with-rheumatoid-arthritis-treated-by-abatacept-data-from-the-french-orencia-in-rheumatoid-arthritis-ora-registry/