Session Information
Date: Monday, November 9, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Bench to Bedside
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose:
Lupus nephritis (LN) is a major complication of systemic lupus erythematous (SLE) and affects ~60% of patients during the course of their disease, leading to significant morbidity and mortality. Previous studies examining the safety of percutaneous kidney biopsy to diagnose LN have found variable complication rates depending on disease type studied, ranging from 4-11% in autoimmune/SLE patients to 15-17% in safety studies of any kidney disease. The purpose of our study was to define the safety of obtaining additional tissue for research during clinically indicated renal biopsies in a SLE cohort.
Methods:
Patients were enrolled across 15 clinical US sites in the SLE Accelerating Medicines Partnership (AMP). Kidney biopsies were clinically indicated to evaluate proteinuria (urine protein creatinine ratio [uPCR] > 0.5). Patients with a history of renal transplant, use of rituximab within 6 months of biopsy, and current pregnancy were excluded. Ultrasound/CT-guided kidney biopsies were performed by interventional radiologists/nephrologists generally using an 18-gauge needle although technique, number of routine passes and core lengths varied. An additional core taken solely for research purposes, or a piece of core with sufficient glomeruli remaining from the routine passes and not required for clinical diagnosis, was collected. All adverse events (AEs) occurring within 30 days of biopsy were reported, including duration, severity, type, and resolution.
Results: 482 patients underwent a renal biopsy between 2014 and 2020. All patients met criteria for SLE (ACR or SLICC) and the majority were female (85%). Pathologic assessment of clinical biopsies revealed ISN/RPS Class I-VI for most biopsies, although 45 biopsies (9%) yielded a non-LN diagnosis (Table 1). Overall, 37 patients (8%) experienced an AE with several more than one, with a total of 41 AEs reported. Of these AEs, 8 (20%) were considered by the site investigator to be unrelated or unlikely to be related (included pain, shortness of breath, cardiac arrest, fall, and hemoglobin decrease due to sepsis) and 33 (80%) were deemed possibly, probably, or definitely related to the study procedure. Of these events, 9/33 (28%) were mild, 10 (30%) were moderate, and 12 (36%) were deemed severe. In 18 patients (4%) the AEs were considered serious as defined by inpatient or prolonged hospitalization, significant incapacity, or requiring intervention to prevent permanent impairment. The most common related AEs were bleed-related complications, including hematoma, hemorrhage, and hemoglobin decrease (N= 29). Of these, 18 required hospitalization, with 4 of these patients receiving a blood transfusion. All 29 bleed-related complications resolved. The length of the research biopsy did not associate with an AE.
Conclusion: Procurement of an additional kidney biopsy core for research purposes in SLE patients undergoing a clinically-indicated kidney biopsy did not result in an increase in adverse events compared to the adverse event rate in prior studies of the safety of percutaneous kidney biopsy. Accordingly, inclusion of a research core should be considered feasible for future studies to advance discovery of new therapeutic targets and prognostic indicators in LN.
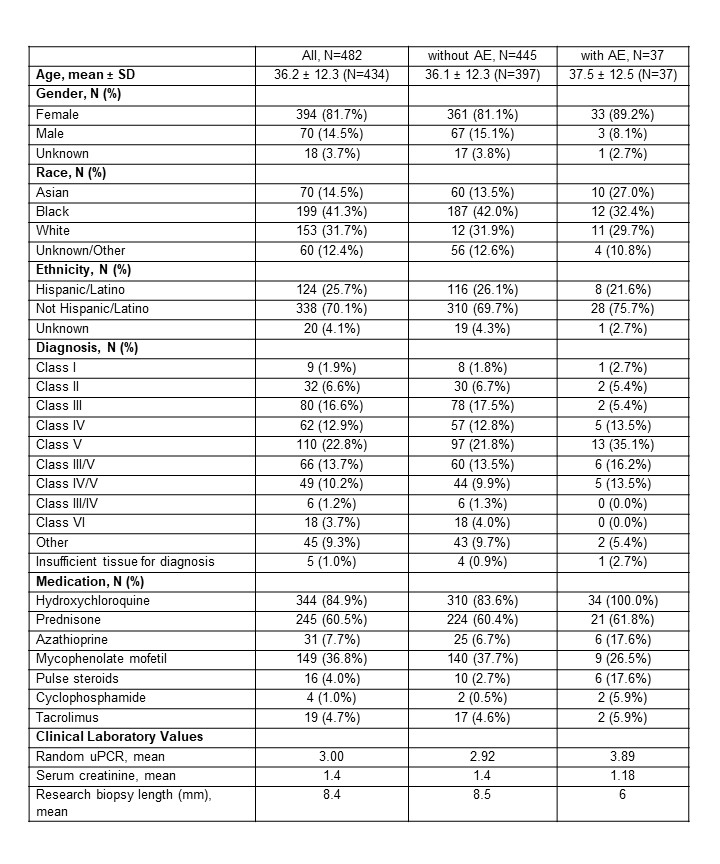 Table 1. Patient characteristics at time of kidney biopsy.
Table 1. Patient characteristics at time of kidney biopsy.
To cite this abstract in AMA style:
Deonaraine K, Carlucci P, Fava A, Li J, Wofsy D, James J, Putterman C, Diamond B, Fine D, Monroy-Trujillo J, Haag K, Apruzzese W, Belmont H, Izmirly P, Connery S, Payan-Schober F, Furie R, Berthier C, Dall'Era M, Cho K, Kamen D, Kalunian K, Accelerating Medicines Partnership in SLE Network T, Petri M, Buyon J. Safety of Obtaining Research Tissue During Clinically Indicated Kidney Biopsies: Data from the Lupus Accelerating Medicines Partnership [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/safety-of-obtaining-research-tissue-during-clinically-indicated-kidney-biopsies-data-from-the-lupus-accelerating-medicines-partnership/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-obtaining-research-tissue-during-clinically-indicated-kidney-biopsies-data-from-the-lupus-accelerating-medicines-partnership/
