Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Janus kinase inhibitor (JAKi) increases the risk of the reactivation of herpes zoster (HZ) virus and may thus be temporarily discontinued in cases of HZ infection. However, the amount of information on the outcomes of JAKi use after an episode of HZ reactivation is insufficient. In this study, we sought to determine the safety of JAKi during or following an HZ reactivation.
Methods: Medical records of all patients (n = 417) who received JAKi at a tertiary referral center between August 2015 and May 2021 were retrospectively reviewed. Among them, data from patients who developed HZ reactivation were collected and the clinical outcomes were evaluated for those who continued or resumed JAKi after HZ reactivation.
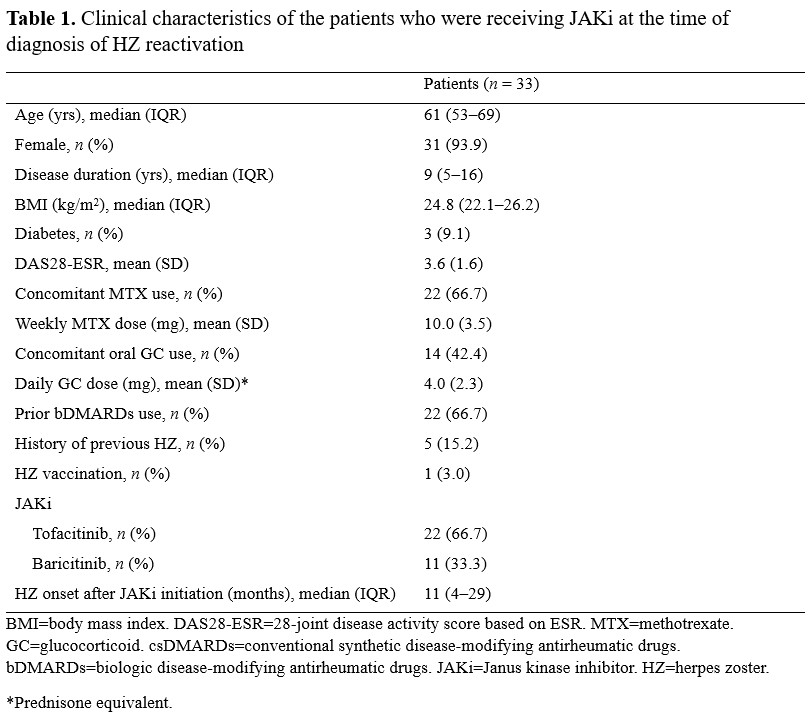
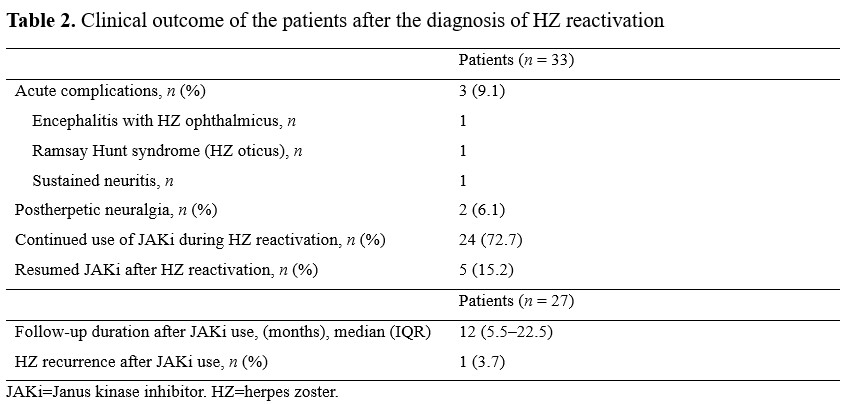
Results: Of 417 patients who received JAKi, 33 (7.9%) developed HZ reactivation during JAKi treatment (tofacitinib, n = 22; baricitinib, n = 11). The median age of the patients (male, n = 2; female, n = 31) was 61 years (IQR, 53–69); 14 patients received glucocorticoids, and the mean dose of prednisone was 4.0 ± 2.3 mg. The median duration of JAKi administration before HZ reactivation was 11 months (IQR, 4–29). JAKi was continued in 24 (72.7%) patients during the HZ reactivation episode, and 5 (15.2%) patients temporarily discontinued the JAKi and then resumed it after episode of HZ. All patients with HZ reactivation had typical skin lesions, and 3 (9.1%) patients had acute complications such as encephalitis with HZ ophthalmicus. Four (12.1%) patients, including the three patients with complications, permanently discontinued JAKi. Of the 27 patients who were followed up for a median of 12 months (IQR, 5.5–22.5) after the HZ reactivation episode, HZ reactivation recurred in one (3.7%) patient; this patient maintained JAKi treatment for further 18 months, during which additional HZ recurrence was not observed. Two (6.1%) patients had postherpetic neuralgia.
Conclusion: JAKi was commonly continued or re-administered in patients with HZ reactivation, and the majority of patients did not experience significant complications or a recurrence of HZ reactivation. Thus, the use of JAKi after HZ reactivation episode may be generally safe and well-tolerated.
To cite this abstract in AMA style:
Choi W, Ahn S, Kim Y, Lee C, Yoo B, Hong S. Safety of JAK Inhibitor in Patients with Rheumatoid Arthritis Who Developed Reactivation of Herpes Zoster Virus After Receiving JAK Inhibitor [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/safety-of-jak-inhibitor-in-patients-with-rheumatoid-arthritis-who-developed-reactivation-of-herpes-zoster-virus-after-receiving-jak-inhibitor/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-jak-inhibitor-in-patients-with-rheumatoid-arthritis-who-developed-reactivation-of-herpes-zoster-virus-after-receiving-jak-inhibitor/