Session Information
Date: Tuesday, November 12, 2019
Title: Miscellanous Rheumatic & Inflammatory Disease Poster III: Autoimmune Conditions and Therapies
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with pre-existing autoimmune diseases (AID) have been traditionally excluded from clinical trials of immune checkpoint inhibitors (ICI), so the data on risk of flare of pre-existing AID with ICI therapy is very limited. As we encounter more patients in clinical practice with pre-existing AID who have been treated with ICI therapy, there is a growing need for understanding the management of autoimmune complications from ICI therapies. The aim of our study was to investigate the safety of immune checkpoint inhibitors in patients who have pre-existing AID.
Methods:
After approval from the University of Iowa Hospitals and Clinics (UIHC) IRB, we performed a retrospective chart review of all the patients with pre-existing AID who received any kind of ICI therapy at UIHC through September 1st, 2018. Data collection included- the nature of the pre-existing AID, treatments previously given or currently being given for the AID, type of ICI therapy given, course and duration of ICI therapy, effects of ICI therapy on the AID, timeline of development of flares of pre-existing AID (if applicable), grade and severity of any new immune related adverse events (irAE’s) seen, progression free survival, overall survival and vital status at the time of last documentation available in the chart. Predictors of developing a flare were determined using logistic regression.
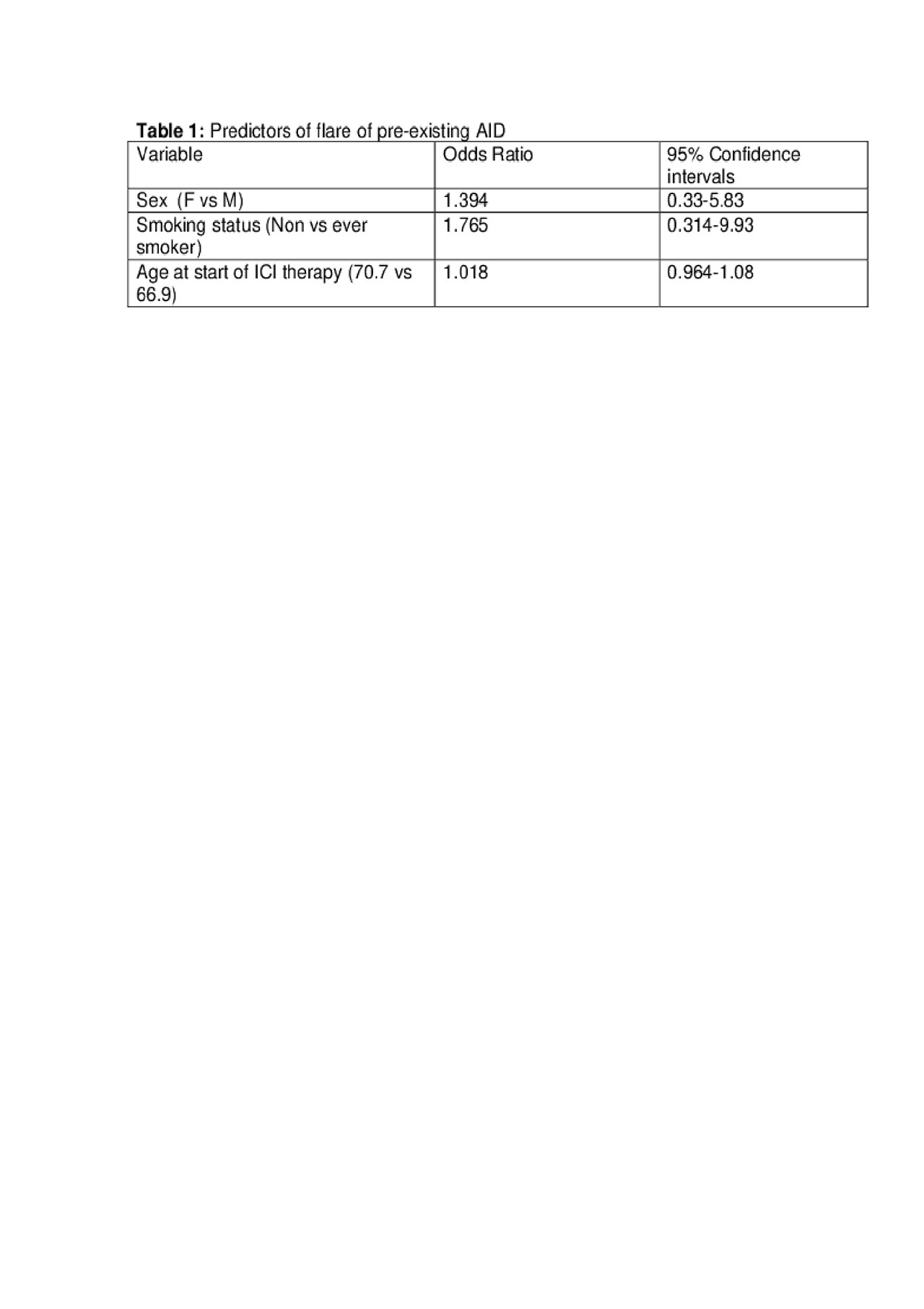
Results: A total of 42 patients with pre-existing AID were identified who received ICI therapy. Out of these, 25 patients had metastatic melanoma, 11 patients had lung cancer, four patients had genitourinary cancer, one patient had diffuse large B cell lymphoma and one patient had head and neck cancer. Twelve patients had flares of their pre-existing AID including three patients with rheumatoid arthritis (RA), two patients with psoriatic arthritis, two patients with polymyalgia rheumatica (PMR), and one patient each with skin psoriasis, Crohn’s disease, bullous pemphigoid, inflammatory arthritis and myasthenia gravis. All of the flares were treated with oral or topical corticosteroids. In addition, one patient with RA flare was treated with tofacitinib and one Crohn’s flare was treated with infliximab. Female sex, older age and non-smoker status were predictors of flare although these were not statistically significant (Table 1). Five patients developed new irAE’s, which were managed with corticosteroids as well. ICI therapy was stopped in four patients due to AID flare but continued in the rest. The median progression-free survival (PFS) in melanoma and lung cancer cohort was 943 and 158 days and median overall survival (OS) was undefined and 361 days respectively.
Conclusion: In patients with pre-existing AID treated with an ICI therapy, flare of AID happened in a small proportion of patients (28%). Even among those who flared, they were managed safely with corticosteroids alone or with additional disease modifying therapies. Immunotherapy could be safely continued in majority of the patients with close monitoring and multidisciplinary collaboration.
To cite this abstract in AMA style:
Kaur A, Swami U, Fatima M, Ginn M, Stein J, Gao Y, Zakharia Y, Singh N. Safety of Immune Checkpoint Inhibitors in Patients Treated for Cancer with Pre-existing Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-of-immune-checkpoint-inhibitors-in-patients-treated-for-cancer-with-pre-existing-autoimmune-diseases/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-immune-checkpoint-inhibitors-in-patients-treated-for-cancer-with-pre-existing-autoimmune-diseases/