Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Filgotinib (FIL) is a Janus kinase (JAK) 1 preferential inhibitor, approved for treatment of moderate to severe active RA in Europe, the UK, and Japan.1,2 Graded laboratory abnormalities from placebo-controlled analyses and long-term data on lymphocytes have been reported previously.3,4 Here we report the effect of FIL on laboratory parameters in the FINCH 4 long-term extension (LTE).
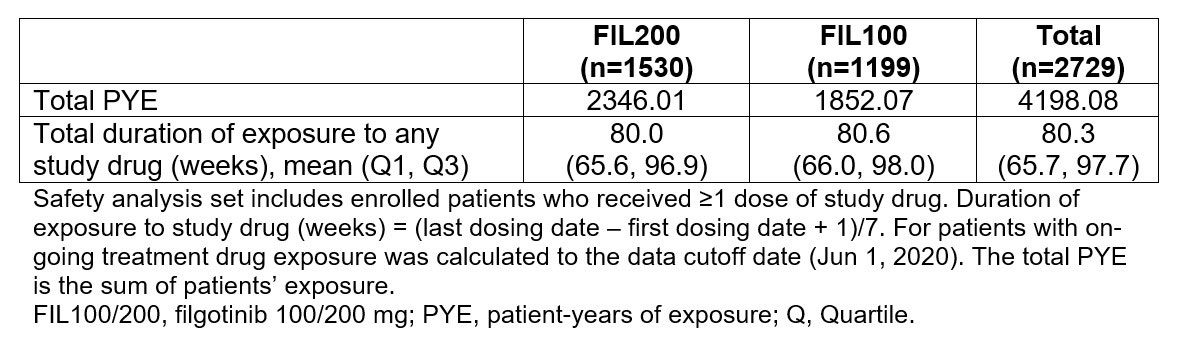
Methods: Safety was assessed from LTE baseline (BL) to data cutoff (Jun 1, 2020) in patients (pts) receiving ≥1 FIL dose (FIL 200 mg [FIL200] or 100 mg [FIL100]) in FINCH 4 (NCT03025308; adults with RA who met ACR criteria for functional class I–III and had completed FINCH 1/2/3). Laboratory abnormalities were graded per Common Terminology Criteria for Adverse Events v4.03. Frequencies and exposure-adjusted incidence rates (EAIRs)/100 pt-years of exposure (PYE) for graded abnormalities are reported. Median laboratory parameters are reported to LTE Week (W) 48.
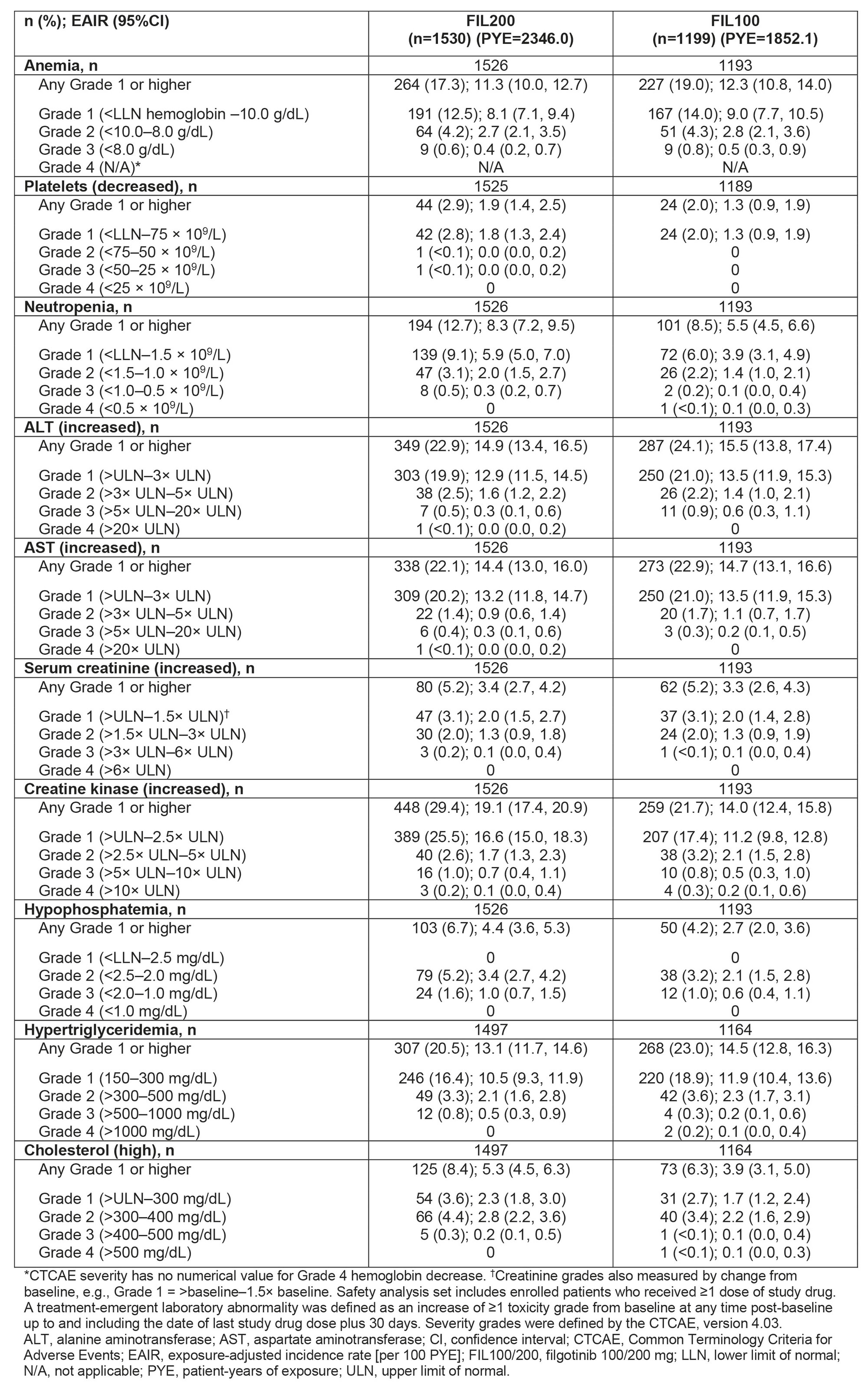
Results: In FINCH 4, 2729 pts received FIL for 4198.08 PYE (mean 80.3 weeks); exposure was similar between dose groups (Table 1). Frequency and graded EAIR of laboratory abnormalities were similar for anemia, decreased platelets, and increased alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum creatinine, and higher in the FIL200 vs FIL100 group for neutropenia, increased creatinine kinase (CK), hypophosphatemia, and cholesterol (high) (Table 2). No Grade 4 decreased phosphate laboratory abnormalities were observed and hypophosphatemia was not associated with adverse events. Laboratory abnormalities led to discontinuation of FIL in 7 pts: ALT and AST increased, 3 (0.2%, FIL 200); ALT increased, 1 (< 0.1%, FIL 100); and neutropenia 3 (0.3%) (FIL 100). From LTE BL to W48, hemoglobin, platelets, ALT, and AST were relatively stable, with no clear differences between doses, or between pts with or without prior FIL. Neutrophil count was relatively stable from LTE BL to W48. Neutrophils decreased for the FIL200 group with no prior FIL exposure, remaining stable from W24. CK and serum creatinine were relatively stable from LTE BL to W48 in pts with prior FIL exposure; in pts with no prior FIL, initial increases plateaued by W6 and W12, respectively, remaining stable. Changes in phosphate levels from LTE BL were small, remaining within normal range (2.2–5.1 mg/dL). Triglycerides were stable over time with no differences between groups. Total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels were stable in pts with prior FIL exposure. In pts with no prior FIL exposure, small increases in total cholesterol, HDL, and LDL plateaued by W24, remaining stable. The LDL:HDL ratio was stable, with no differences between groups (Figure).
Conclusion: Laboratory abnormalities were generally mild to moderate, similar to previous observations.3 Frequency and EAIR were higher in the FIL200 vs FIL100 group for neutropenia, increased CK, hypophosphatemia, and high cholesterol.
References:
- Jyseleca SmPC. Galapagos NV; May 2022
- Jyseleca Japanese PI. Gilead Sciences K.K.; Sep 2020
- Winthrop KL, et al. Ann Rheum Dis 2022;81:184–92
- Gottenberg JE et al. Ann Rheum Dis 2022;81(suppl 1):POS0513
To cite this abstract in AMA style:
Buch M, Galloway J, Favalli E, Constantin A, Durez P, Van Hoek P, Watson C, Stiers P, Rajendran V, Van Beneden K, Takeuchi T, COMBE B. Safety of Filgotinib in Patients with RA: Laboratory Analysis Results from a Long-Term Extension Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/safety-of-filgotinib-in-patients-with-ra-laboratory-analysis-results-from-a-long-term-extension-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-filgotinib-in-patients-with-ra-laboratory-analysis-results-from-a-long-term-extension-study/