Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Since Immune Checkpoint Inhibitors (ICI) revolution, oncologists face immune-related adverse events (irAEs) including rheumatologic irAEs with inflammatory arthritis. Treatment of rheumatologic irAE can be challenging, using glucocorticosteroids (GC), conventional synthetic (cs-) or biological (b-) DMARDs without impeding ICI antitumoral efficiency. The objective of our study was to assess the safety of current rheumatologic irAE treatments.
Methods: We conducted a monocentric retrospective observational study at the Centre Hospitalier Lyon Sud, Hospices Civils de Lyon, Lyon, France. Eligible patients were adult patients treated with ICI presenting rheumatologic irAE. Patients with active inflammatory rheumatologic disease and who were not assessed by a rheumatologist were excluded. Data were obtained from medical charts using a standardized data collection. Kruskal-Wallis and Wilcoxon tests were used for comparing continuous variables. Fisher’s exact test was used for comparison between categorical variables. Spearman coefficient was used for correlation test. Survival analysis was performed using Kaplan Meier method.
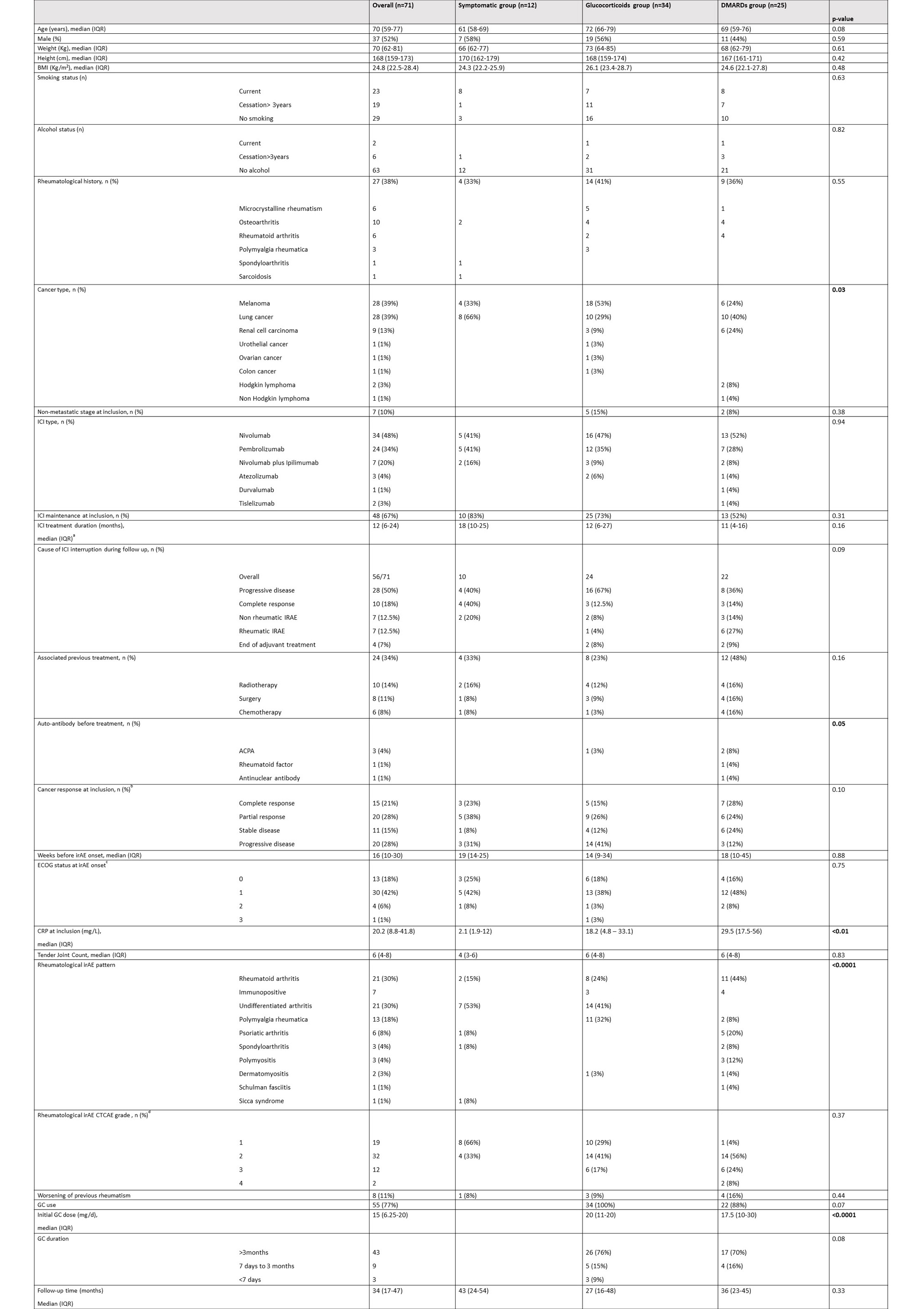
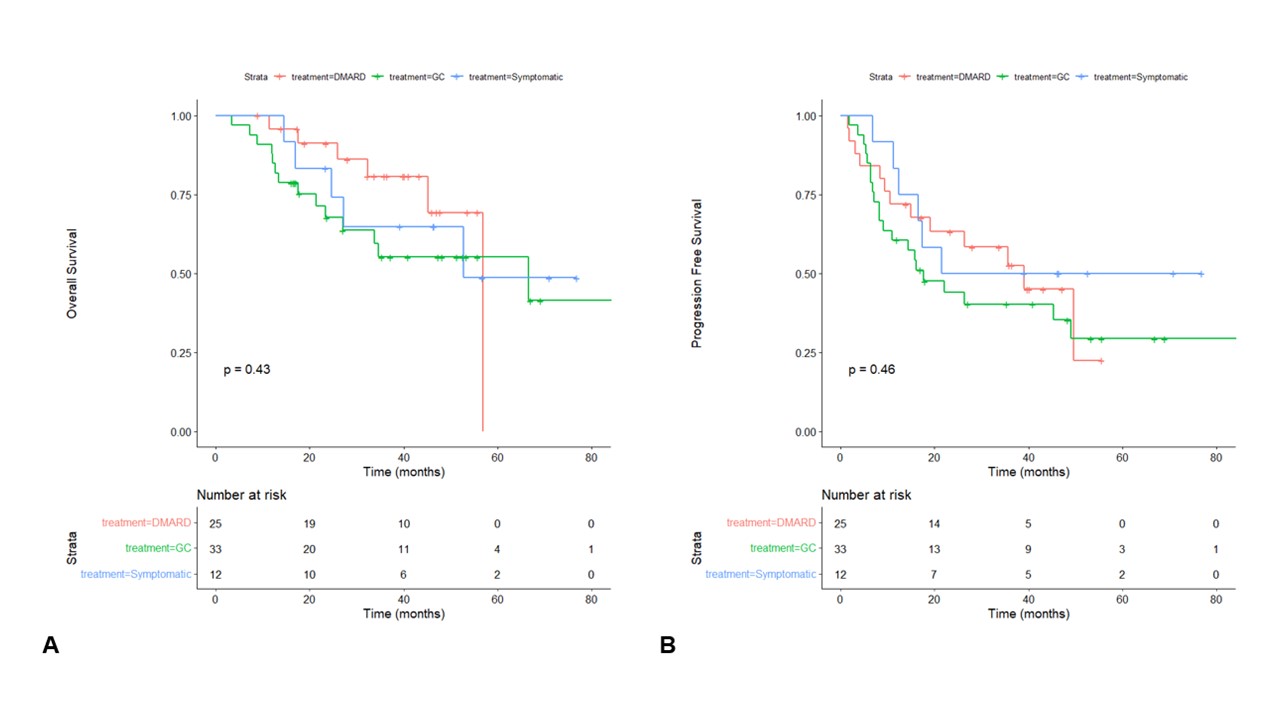
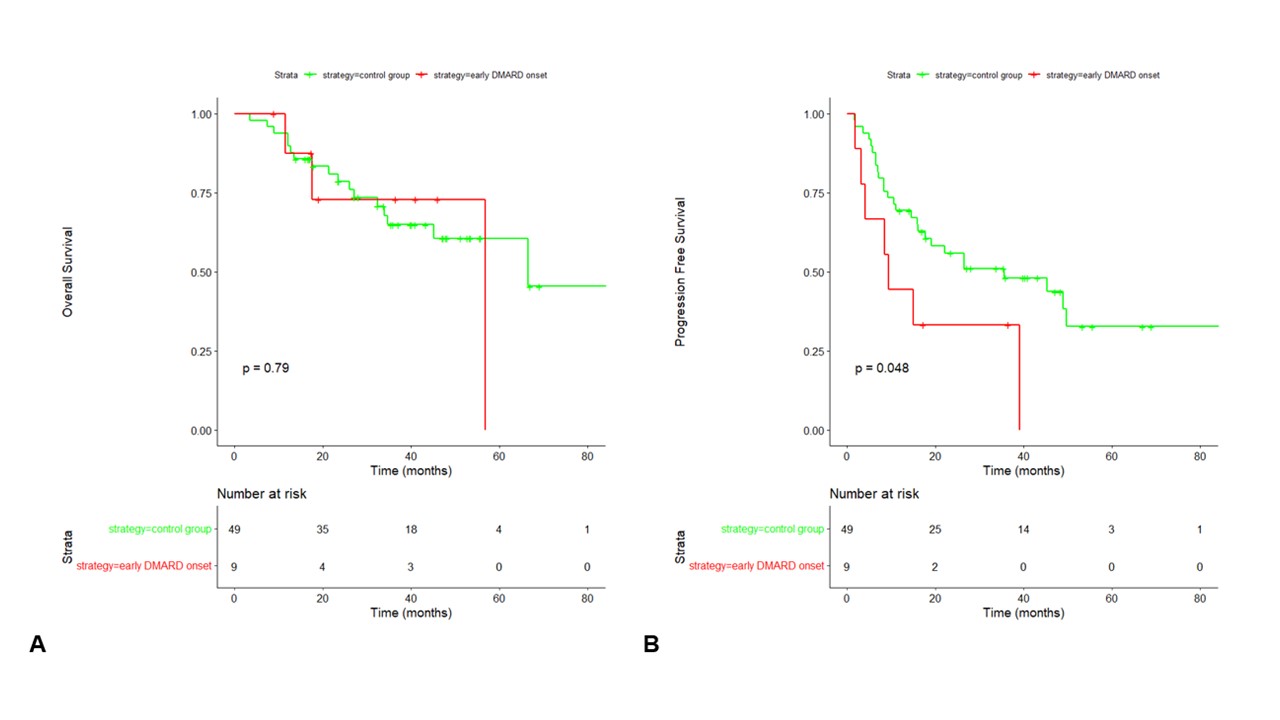
Results: Between July 2016 to October 2022, 71 patients were included (Table 1). Patients were allocated to 3 groups regarding the treatment of their rheumatologic irAE: symptomatic group when treated with analgesics or NSAIDs (n=12), GC group when treated with systemic corticosteroids (n=34) and DMARD group when treated with csDMARD and/or bDMARD (n=25). Eighteen (72%) patients were treated with methotrexate. Ten patients were treated with bDMARD: 5 with infliximab (20%), 4 with tocilizumab (16%), 1 with etanercept (4%). No significant difference was found between different groups (Figure 1) regarding overall survival (OS, p=0.43), and progression free survival (PFS, p=0.46). We realized subset survival analysis including GC group, patients treated csDMARD alone (n=15) and patients treated with csDMARD and/or bDMARD (n=10), with no difference for OS and PFS. However, OS was positively correlated with time between irAE and bDMARD onset (R=0.88, p< 0.0001) unlike other groups. Based on this result, we compared patients treated with early DMARD onset (< 6 weeks after irAE onset, n=9) with a control group composed of patients with later DMARD onset after irAE and patients treated with GC (n=50). Patients in early DMARD group were mostly treated with csDMARD: 5 with methotrexate, 2 with immunoglobulins, 1 with hydroxychlororquine. One patient was treated with tocilizumab alone. Survival analysis realized between early DMARD group and control group (Figure 2) did not show significant difference for overall survival (p=0.79), but progression free survival was lower in early DMARD group than in control group (p=0.048).
Conclusion: Our data support concerns previously raised about cancer progression in irAE treated with bDMARD and suggest precautions for using DMARD during the first 6 weeks after rheumatologic irAE onset.
a. 1 missing data. b. 5 missing data. c. 23 missing data. d. 6 missing data. Results are presented with median and interquartile range.
To cite this abstract in AMA style:
Seiller J, Massy E, Peron J, Confavreux C. Safety of DMARDs in Rheumatologic Immune-related Adverse Events Associated with Immune Checkpoint Inhibitors: Data from a Monocentric Cohort in Hospices Civils De Lyon [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-of-dmards-in-rheumatologic-immune-related-adverse-events-associated-with-immune-checkpoint-inhibitors-data-from-a-monocentric-cohort-in-hospices-civils-de-lyon/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-dmards-in-rheumatologic-immune-related-adverse-events-associated-with-immune-checkpoint-inhibitors-data-from-a-monocentric-cohort-in-hospices-civils-de-lyon/