Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: HZ/su has been in use since 2017 and is recommended by the advisory committee on immunization practices (ACIP) as the preferred shingles vaccine due to more than 90% efficacy in preventing shingles and postherpetic neuralgia. Centers for Disease Control and Prevention (CDC) recommends that healthy adults 50 years and older get two doses of HZ/su separated by 2 to 6 months. However, patients with immune-mediated inflammatory diseases were all excluded from HZ/su trials. HZ/su is reported to have high reactogenicity due to the presence of a novel adjuvant which has never been used before in humans. Given the high immunogenicity, rheumatologists are concerned about the aggravation or potentially induction of autoimmune inflammatory diseases. Given the sparsity of data regarding the safety of HZ/su among rheumatology patients, we looked at the safety of the vaccine among subjects who are being treated with synthetic and biologic DMARDs.
Methods: This a retrospective study involving subjects from university-affiliated rheumatology practice who received HZ/su in 2018. Data were extracted from the electronic medical record. In addition to demographic details and medication use, Rapid 3 score and CRP level (mg/dl) before and after the series of vaccine administration were recorded. Any patient-reported adverse reactions related to HZ/su and provider documented flare-up of disease, after vaccination, were also recorded. Statistical analysis using paired T-test was conducted in order to see if there was a difference between Rapid 3 and CRP scores before and after vaccination. The analysis was done with SPSS 25.0.
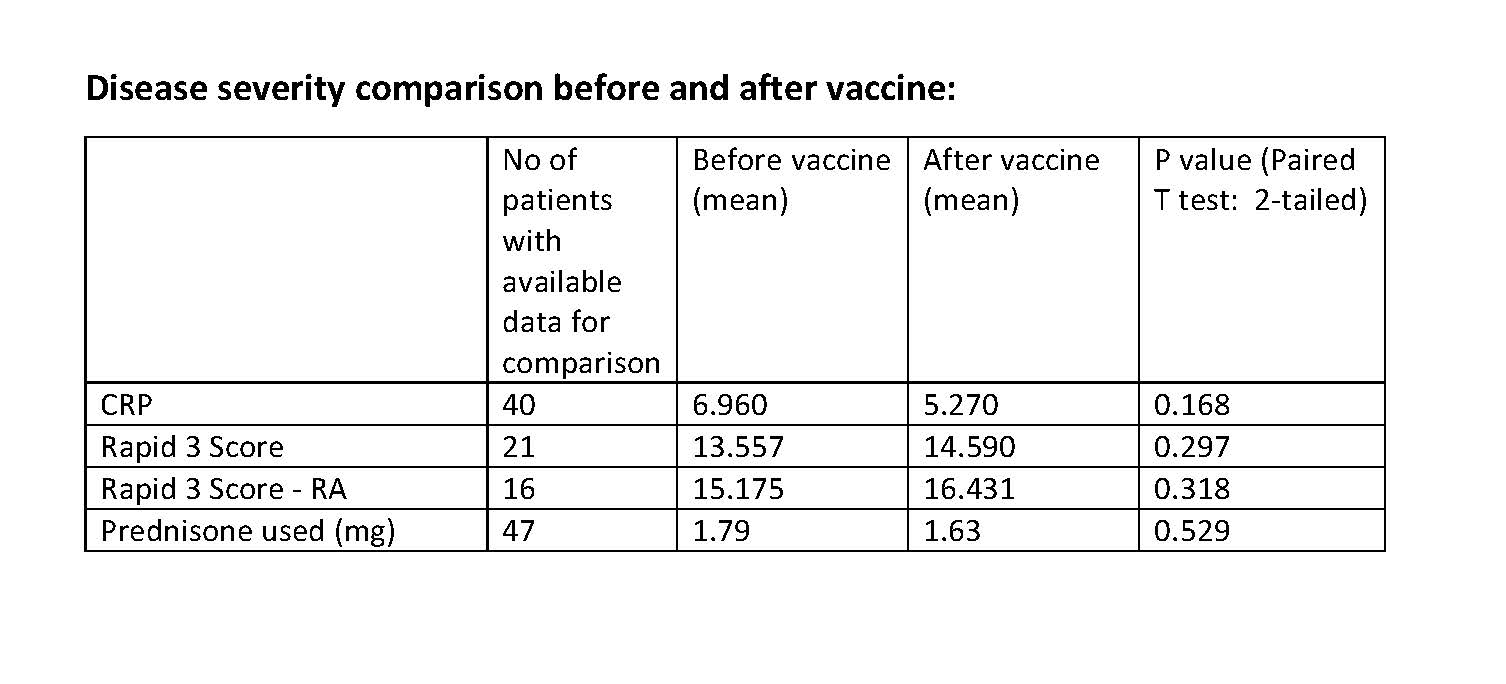
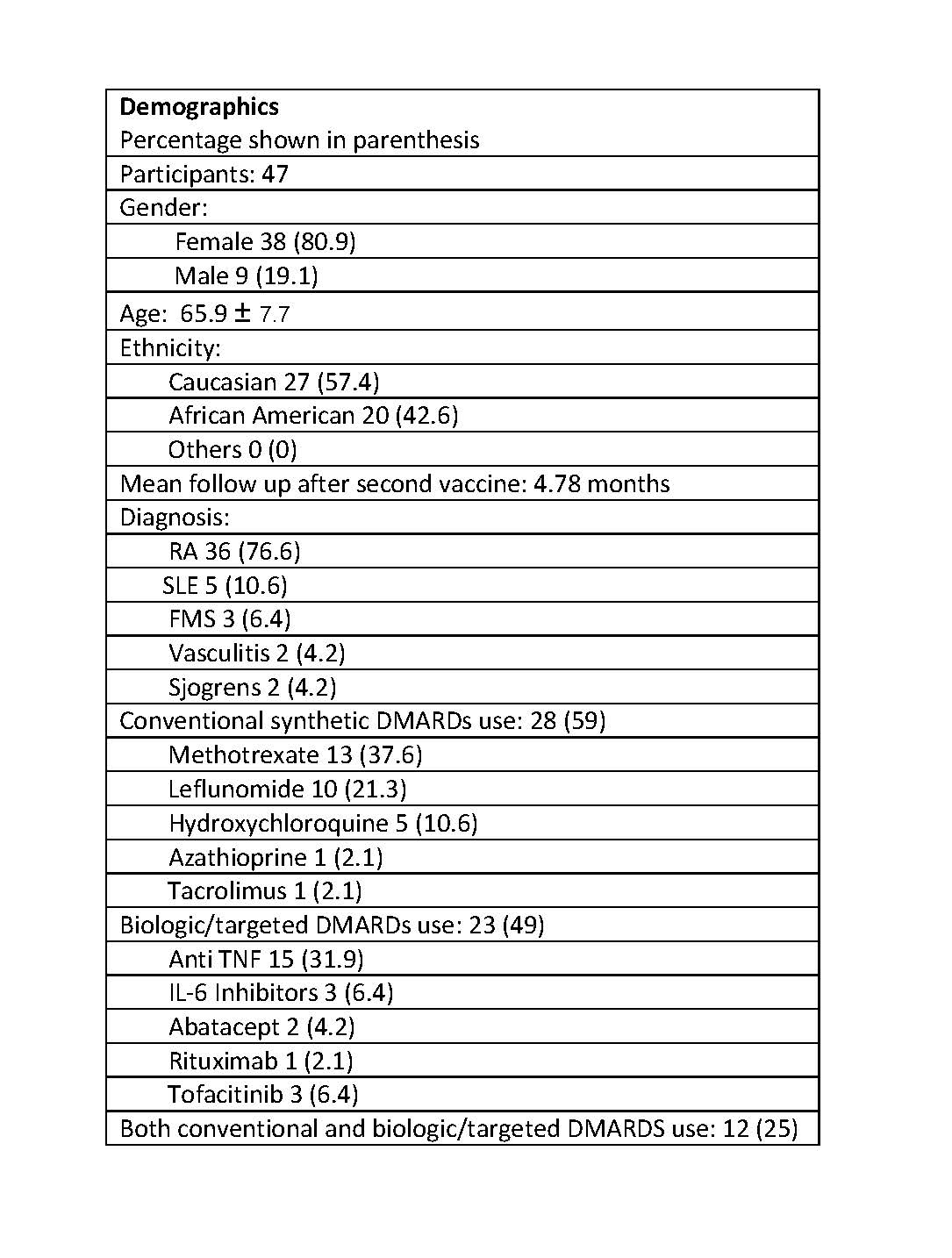
Results: A total of 47 patient had received both doses of HZ/su. The mean age of patients was 65.9 and of all 76.6 % of the patients had rheumatoid arthritis as a primary diagnosis. The CRP, Rapid 3 for all patients, and Rapid 3 for RA patients were compared before and after vaccinations and were found to have no statistically significant differences. Average prednisone dose was similar before and after vaccination. Of all patients, 6.4% reported adverse reactions. Adverse reactions were non-severe and included fever, myalgia, fatigue, and stomach upset. None of the adverse events led to hospital admission, major organ failure or death. There were 4 events of underlying rheumatic disease flare-ups. All of the 4 patients had RA, and the 2 had self-discontinued the DMARDs (Methotrexate and Leflunomide). There was one event of herpes zoster occurred in a patient with SLE on Hydroxychloroquine and rituximab after receiving the first dose of vaccine.
Conclusion: HZ/su was well tolerated in our subjects. There was no statistically significant difference in CRP values in all patients, Rapid 3 scores in all patients, and Rapid 3 scores among rheumatoid arthritis patients before and after the HZ/su.
To cite this abstract in AMA style:
Acharya S, Raza S, Pattanaik D, Howard A. Safety of Adjuvanted Herpes Zoster Subunit Vaccine (HZ/su, Shingrix) Among Patients with Autoimmune Inflammatory Diseases [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-of-adjuvanted-herpes-zoster-subunit-vaccine-hz-su-shingrix-among-patients-with-autoimmune-inflammatory-diseases/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-adjuvanted-herpes-zoster-subunit-vaccine-hz-su-shingrix-among-patients-with-autoimmune-inflammatory-diseases/