Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
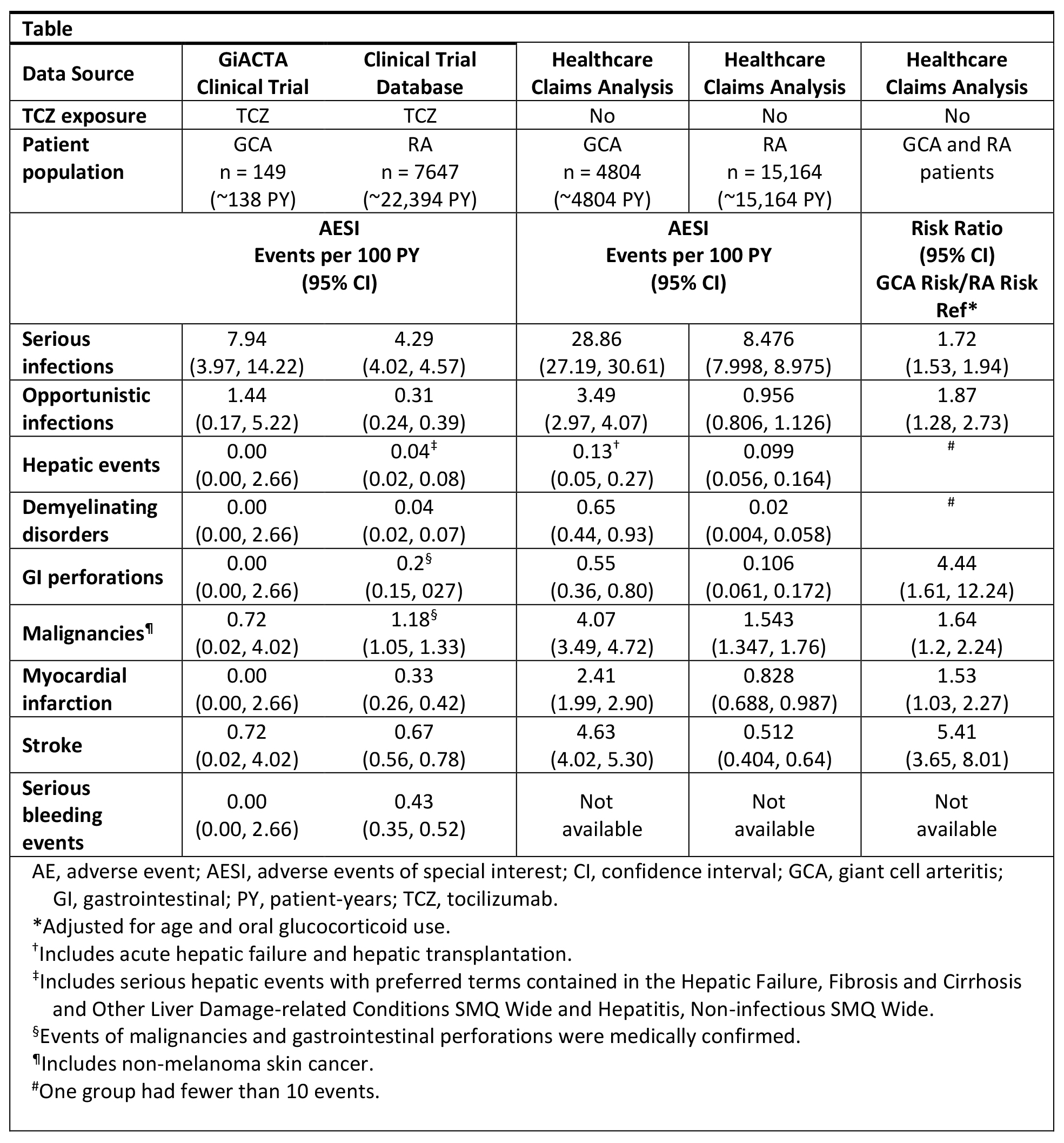
Background/Purpose: Recent clinical trial findings have shown efficacy for tocilizumab (TCZ) in treating giant cell arteritis (GCA).1,2 TCZ was approved for the treatment of GCA in the US in 2017 and of rheumatoid arthritis (RA) in the EU in 2009 and in the US in 2010, and it has a well-characterized safety profile in RA patients (pts). However, the safety profile of TCZ in GCA pts may differ from that observed in RA pts due to differences in the underlying disease and the higher dosing of glucocorticoids (GCs) used to treat GCA. This study describes the incidence rates (IRs) of safety events in GCA pts or RA pts in a general healthcare claims database and in the TCZ clinical development program to contextualize the observed safety profile of TCZ in GCA.
Methods: Adverse events of special interest (AESI) IRs for TCZ in adult GCA and RA pts were estimated from a US-based MarketScan administrative healthcare claims database. GCA pts were ≥50 years and received ≥2 prescriptions for oral GCs (the first within 6 months of diagnosis); for comparability, only RA pts who were ≥50 years were included. AESI included infections, hepatic events, gastrointestinal (GI) perforation, demyelination, cardiovascular events, bleeding events, and malignancies. AESI IRs were also calculated for a pooled population of RA pts who received TCZ in clinical trials and for GCA pts who received TCZ in the GiACTA clinical trial.1-3 Risks for AESI among GCA pts vs RA pts in the claims database, adjusted for age and oral GC use, were estimated with Poisson regression.
Results: The healthcare claims database included 4804 GCA pts (mean [SD] age, 73.4 [9.8] years; follow-up, 3.89 [3.12] years) and 15,164 RA pts (mean [SD] age, 60.3 [8.17] years; follow-up, 4.52 [3.78] years). IRs of myocardial infarction, stroke, GI perforation, and hepatic events in TCZ-naive GCA pts from the healthcare claims database (Table) exceeded IRs in TCZ-naive RA pts from healthcare insurance claims. Consistent with this, AESI IRs in TCZ-treated GCA pts (n = 149, ~138 PY) in the GiACTA clinical trial were different from those in TCZ-treated RA pts (n = 7647; mean [SD] age, 52 [12.6] years; ~22,394 PY) in the pooled clinical trial population. GCA pts were at higher risk than RA pts for all AESI in the US claims database after adjusting for age and oral GC use.
Conclusion: TCZ-naive GCA pts from the healthcare claims analysis had higher AESI IRs than TCZ-naive RA pts. Clinical trial data for TCZ in RA pts and GCA pts reflect these findings and suggest differences in the underlying disease burdens associated with RA and GCA. The higher doses and longer courses of GCs used in GCA may also influence the incidence of AESI. Future analyses are needed to determine how differences in patient demographics may further differentiate the safety profile of TCZ between RA pts and GCA pts. References: 1Stone H et al. Arthritis Rheumatol. 2016;68:S10 abstr. 2Stone JH et al. N Engl J Med. In press. 3Villiger PM et al. Lancet. 387(10031):1921.
To cite this abstract in AMA style:
Gale S, Dimonaco S, Trinh H, Tuckwell K, Collinson N, Stone JH, Sarsour K, Pei J, Best JH, Birchwood C, Mohan S. Safety Events in Giant Cell Arteritis and Rheumatoid Arthritis Patient Populations [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/safety-events-in-giant-cell-arteritis-and-rheumatoid-arthritis-patient-populations/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-events-in-giant-cell-arteritis-and-rheumatoid-arthritis-patient-populations/