Session Information
Date: Monday, November 18, 2024
Title: Abstracts: SLE – Treatment II: Non-Cellular Lupus Therapeutics
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: There is strong evidence for the relationship between Toll-like receptor (TLR)7/8 and systemic lupus erythematosus (SLE) pathophysiology. Dual inhibition of TLR7/8 will modulate innate immune responses and suppress the production of many inflammatory cytokines, including IFN α. We developed E6742, a novel small molecular with selective TLR7/8 dual antagonist, and its safety, tolerability, pharmacokinetics (PK), biomarker response, and efficacy were assessed in this Phase 1/2, randomized, double-blind, placebo-controlled study in SLE for the first time (NCT05278663).
Methods: SLE patients who met the 1997 ACR criteria for SLE or the 2019 EULAR/ACR criteria for SLE received 100 mg E6742 or placebo twice daily in cohort 1, and 200 mg E6742 or placebo twice daily in cohort 2. In each cohort, patients were randomized to either E6742 or placebo in a ratio of 2:1 and received multiple oral administrations of E6742 or placebo for 12 weeks. The primary endpoint was to evaluate the safety and tolerability of E6742, the secondary endpoints were to evaluate the PK and interferon gene signature (IGS) after the treatment of E6742.
Results: A total of 12 patients (8 for E6742 100 mg, 4 for placebo) were enrolled in the cohort 1 and subsequently 14 patients (9 for E6742 200 mg, 5 for placebo) were enrolled in the cohort 2. Demographic and baseline characteristics were generally balanced between placebo and E6742 groups.
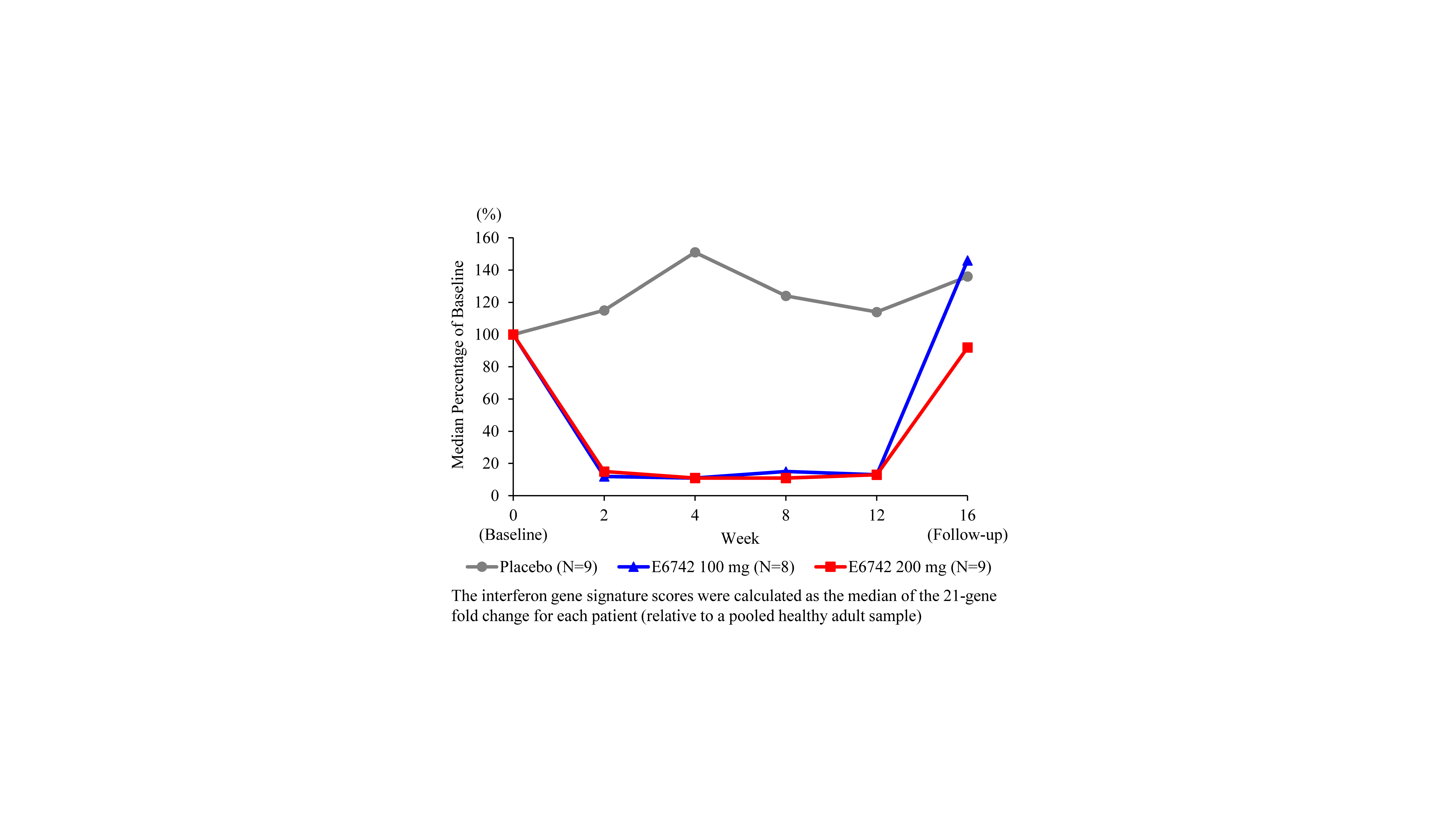
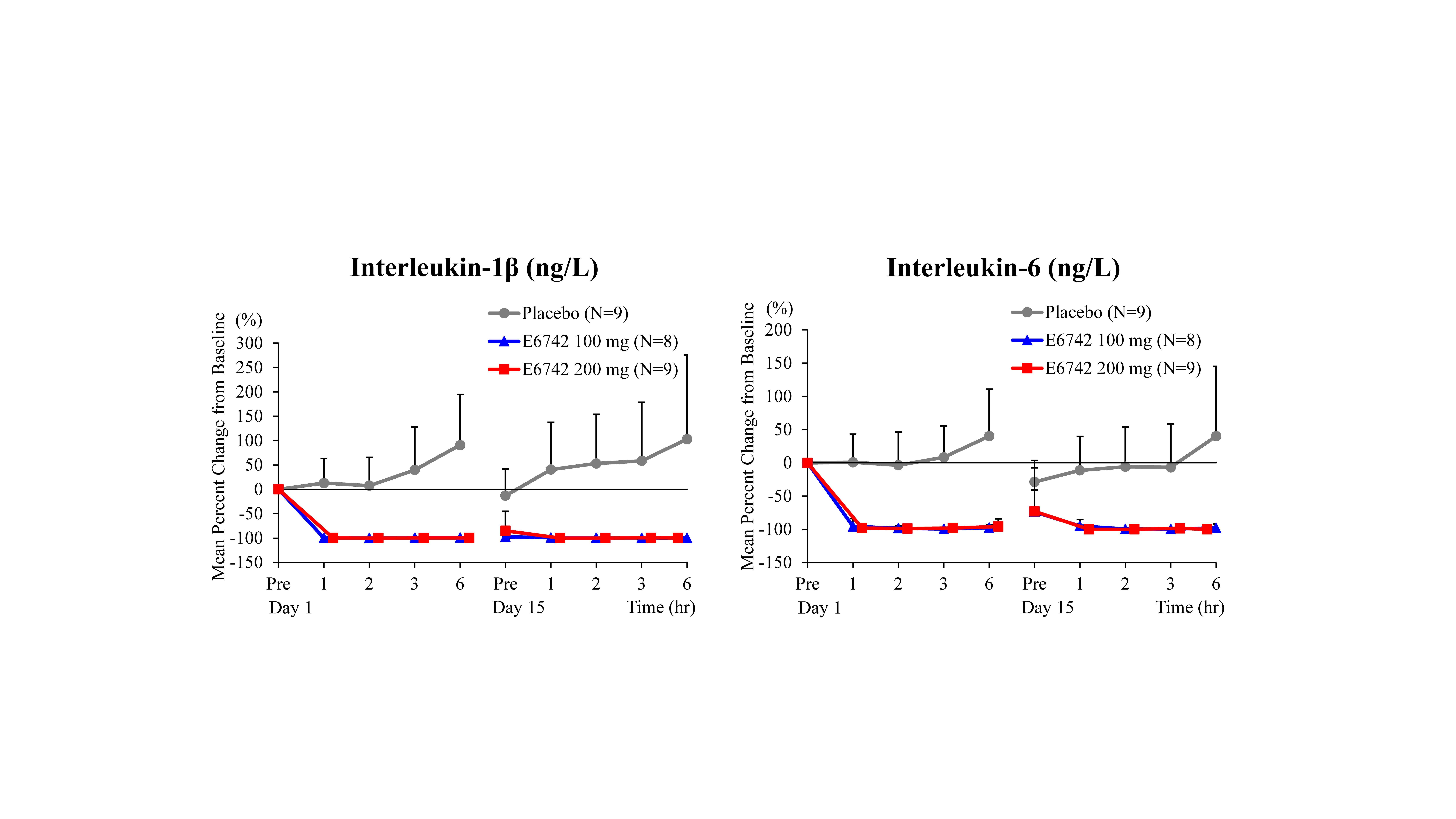
E6742 demonstrated a favorable safety profile and was well tolerated vs placebo. The proportion of patients with any adverse events (AEs) was 58.8 % in the E6742 group (37.5% for 100 mg, 77.8% for 200 mg) and 66.7% in the placebo group. No AEs ≥ Grade 3 or deaths were observed in the study. After oral administration of E6742, plasma concentrations of E6742 increased in dose dependent manner. The IGS and production levels of proinflammatory cytokines (interleukin-1β, interleukin-6) after ex-vivo challenge with a TLR 7/8 agonist were immediately decreased by E6742 treatment. Furthermore, whole transcriptome analysis in this study confirmed that E6742 specifically inhibited only interferon-related pathways, with no nonspecific responses.
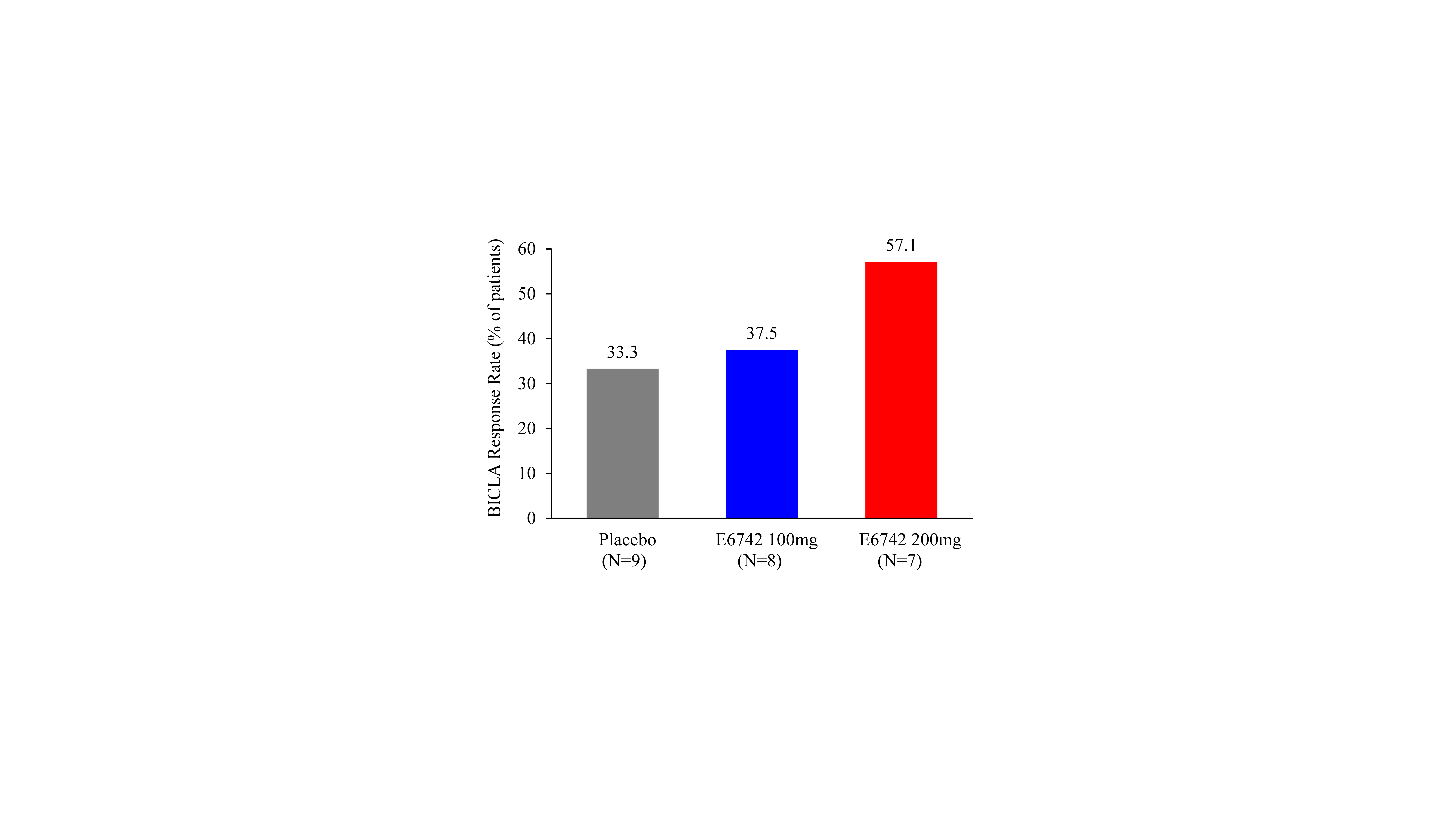
Dose-dependent improvements in the British Isles Lupus Assessment Group-based Composite Lupus Assessment response were observed at Week 12 in the E6742 (37.5% for 100 mg; 57.1% for 200 mg) and placebo (33.3%) groups. E6742 also had therapeutic effects on other symptoms, including skin inflammation, arthritis, and levels of anti-double-stranded DNA antibodies and complements.
Conclusion: E6742 had a favorable safety profile and was well tolerated, with marked IGS responses, proinflammatory cytokines responses and sufficient efficacy signals in patients with SLE. These results provide the first clinical evidence to support E6742 in the treatment of SLE, and support larger, longer-term clinical trials.
To cite this abstract in AMA style:
Tanaka Y, Kumanogoh A, Atsumi T, Ishii T, Tago F, Aoki M, Yamamuro S, Sakayori K, Takahashi K, Akira S. Safety, Biomarker Response, and Efficacy of E6742, a Dual Antagonist of Toll-Like Receptor 7 and 8, in a First-in-Patient, Randomized, Double-Blind, Phase1/2 Study in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-biomarker-response-and-efficacy-of-e6742-a-dual-antagonist-of-toll-like-receptor-7-and-8-in-a-first-in-patient-randomized-double-blind-phase1-2-study-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-biomarker-response-and-efficacy-of-e6742-a-dual-antagonist-of-toll-like-receptor-7-and-8-in-a-first-in-patient-randomized-double-blind-phase1-2-study-in-systemic-lupus-erythematosus/