Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The efficacy and safety of nintedanib have been investigated in patients with systemic sclerosis-associated ILD (SSc-ILD) in the SENSCIS trial and in patients with progressive fibrosing ILDs other than idiopathic pulmonary fibrosis in the INBUILD trial. We used data from these trials to characterize the safety and tolerability of nintedanib in patients with autoimmune disease-related ILDs.
Methods: In both the SENSCIS and INBUILD trials, patients were randomized to receive nintedanib 150 mg bid or placebo. Dose reductions to 100 mg bid and treatment interruptions were permitted to manage adverse events. For this post-hoc analysis, data were pooled from all patients in the SENSCIS trial and patients with autoimmune disease-related ILDs in the INBUILD trial. Adverse events reported by investigators, irrespective of causality, over 52 weeks were analyzed. Analyses were descriptive.
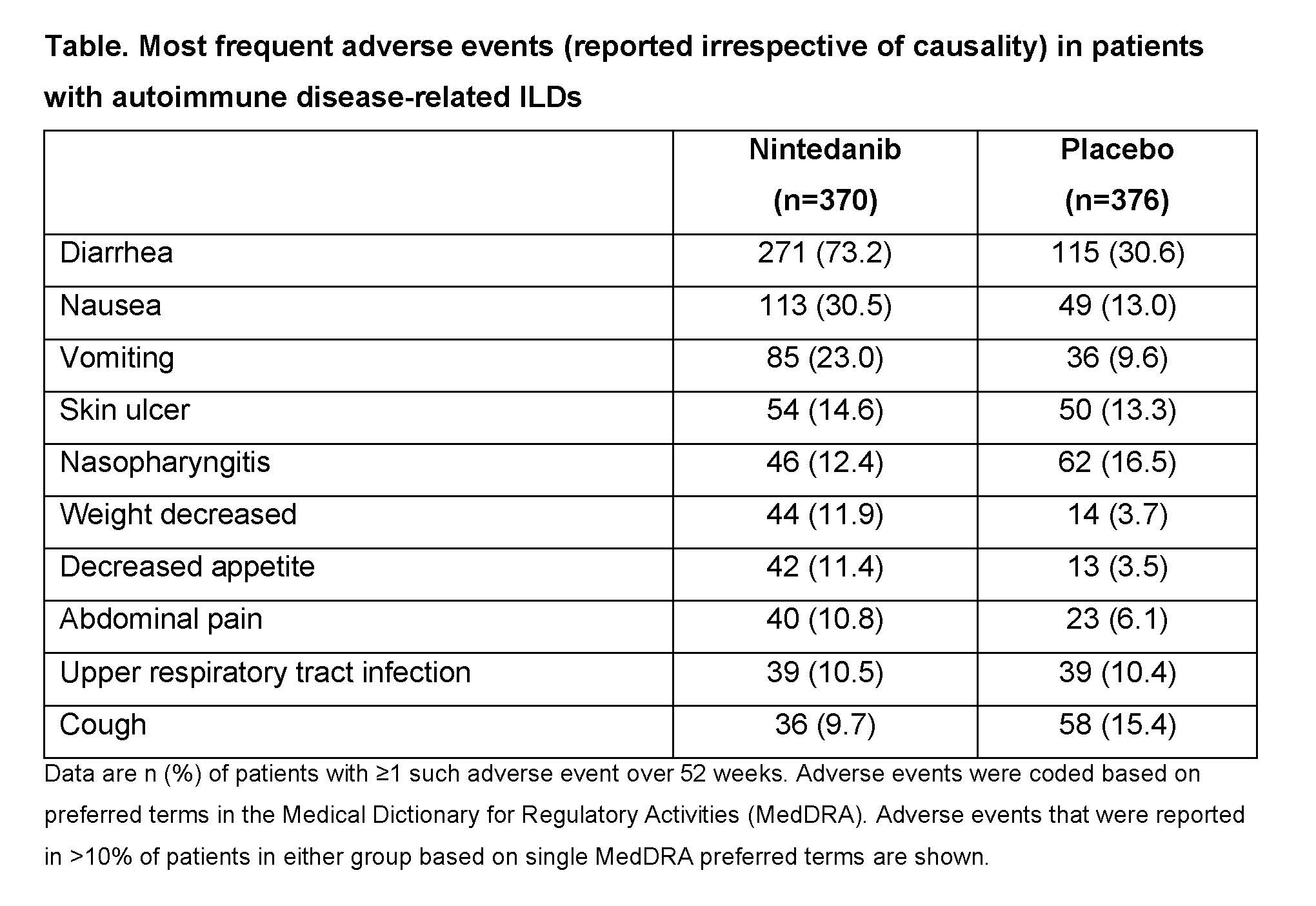
Results: The pooled data set comprised 746 patients with autoimmune disease-related ILDs (615 SSc-ILD, 89 RA-ILD, 19 MCTD-ILD, 23 other ILDs), of whom 370 received nintedanib and 376 received placebo. Mean (SD) exposure to trial medication was 10.4 (3.6) months in the nintedanib group and 11.3 (2.6) months in the placebo group. In the nintedanib and placebo groups, respectively, serious adverse events were reported in 26.2% and 23.9% of patients and fatal adverse events in 2.2% and 2.1% of patients. Adverse events led to dose reduction in 32.2% of patients treated with nintedanib and 3.2% of patients in the placebo group. Adverse events led to permanent treatment discontinuation in 16.5% of patients treated with nintedanib and 9.0% of patients in the placebo group. The most frequent adverse event was diarrhea, which was reported in 73.2% of patients treated with nintedanib and 30.6% of patients in the placebo group (Table). Diarrhea led to permanent treatment discontinuation in 6.8% and 0.5% of patients in the nintedanib and placebo groups, respectively. Based on a standardized MedDRA query, liver-related investigations, signs and symptoms were reported in 16.8% of patients in the nintedanib group and 4.5% of patients in the placebo group. In the nintedanib and placebo groups, respectively, major adverse cardiovascular events were reported in 2.7% and 1.9%, myocardial infarction in 0.3% and 0.8%, bleeding in 11.6% and 8.2%, and gastrointestinal perforation in 0% and 0.3% of patients.
Conclusion: In patients with autoimmune-disease related ILDs, the adverse events associated with nintedanib were characterized predominantly by gastrointestinal events and were managed without treatment discontinuation in most patients. The adverse event profile of nintedanib in patients with autoimmune disease-related ILDs was consistent with that observed in patients with other ILDs.
To cite this abstract in AMA style:
Smith V, Assassi S, Allanore Y, Loaiza L, Tschoepe I, Kanakapura M, Volkmann E. Safety and Tolerability of Nintedanib in Patients with Autoimmune Disease-Related Interstitial Lung Diseases: Pooled Data from the SENSCIS and INBUILD Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/safety-and-tolerability-of-nintedanib-in-patients-with-autoimmune-disease-related-interstitial-lung-diseases-pooled-data-from-the-senscis-and-inbuild-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-tolerability-of-nintedanib-in-patients-with-autoimmune-disease-related-interstitial-lung-diseases-pooled-data-from-the-senscis-and-inbuild-trials/