Session Information
Date: Saturday, November 6, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster I (0387–0413)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Vasculopathy is a crucial feature of systemic sclerosis (SSc). Raynaud’s phenomenon (RP) and digital ulcers (DU) greatly impact the patients’ quality of life. Intravenous (IV) iloprost is broadly used to treat RP and DU secondary to SSc. However, no internationally accepted standardised protocol on iloprost use is currently available.
Methods: We reviewed the clinical records of patients with SSc-related DU and/or moderate-to-severe RP (more than two attacks/day with at least moderate pain) not responsive to calcium channel blockers (CCB), receiving or that have received IV iloprost infusions from January 1st 2011 to March 31st 2021. There were no restrictions concerning combination treatments or comorbidities. Our protocol for IV iloprost consists of an initial ten-hour infusion of iloprost 200ng/mL (0.05 mg in 250 ml of 0.9% saline solution) on five consecutive days, followed by a single-day infusion every month. Over the ten hours of infusion, there is a progressive increase of the dose, up to the patient’s maximum tolerated dose, ranging from 0.5 to 1.5 ng/kg/min. The infusion rate starts at 4 ml/h and is increased according to the following scheme: 4 mL/h (1st hour), 8 mL/h (2nd hour), 12 mL/h (3rdhour), and then 16 mL/h if tolerated by the patient, until the end of the infusion. Adverse events were assessed by consulting clinical records.
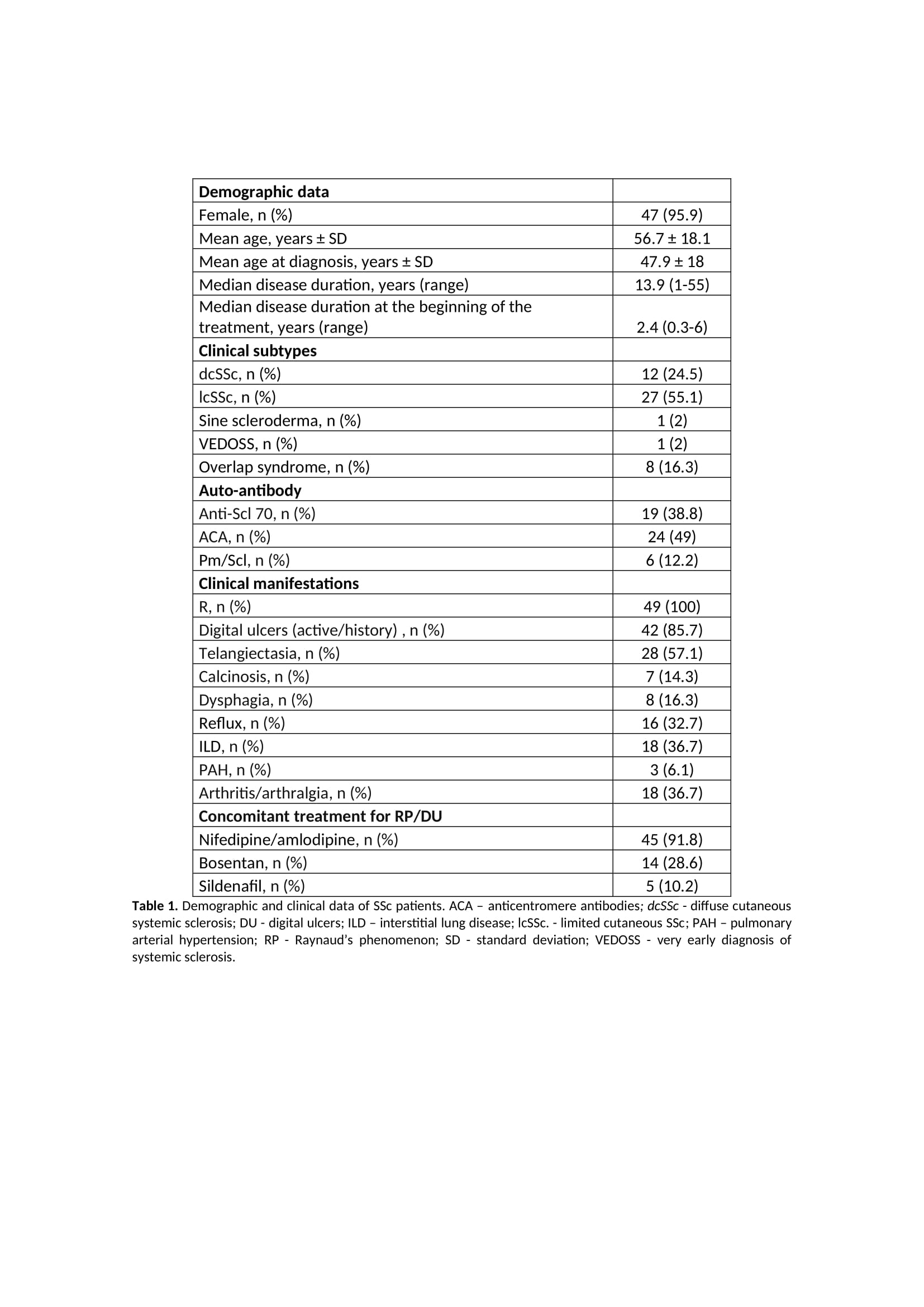
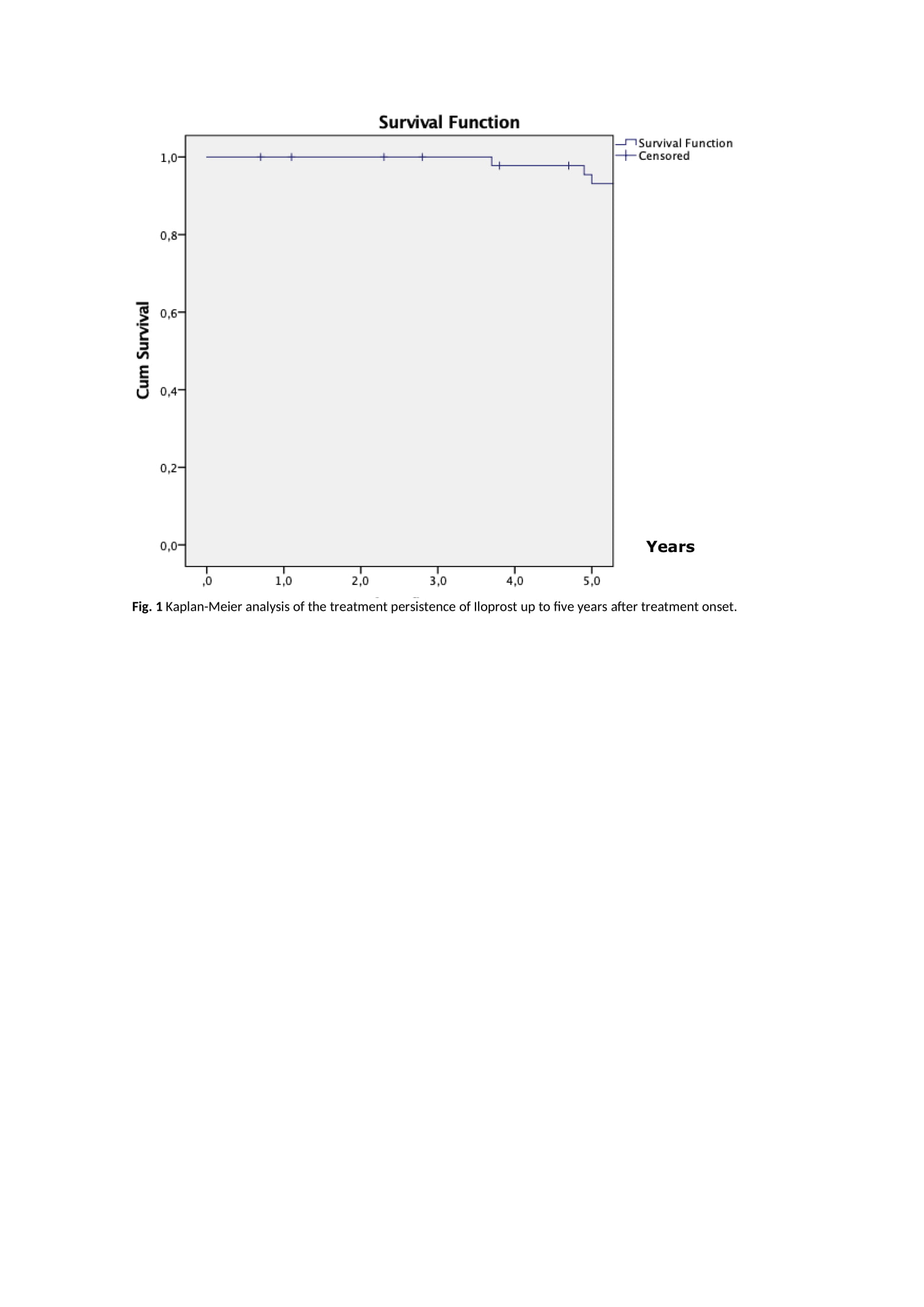
Results: Forty-nine patients with SSc have been treated with IV iloprost according to our treatment protocol. Patients’ characteristics and clinical features are presented in Table 1. Thirty patients initiated iloprost to treat DUs, 14 to treat RP and 5 to treat both. Sixty per cent of patients in the DU group resolved the DUs within the first month of therapy. RP significantly improved in 64% of patients in the RP group within a month. In the RP+DU group, 60% of patients resolved the DUs and significantly improved RP after three months. Currently, 36 patients are actively undergoing treatment. The reasons for discontinuation in the remaining 13 patients included clinical improvement (N=5), switch to treatment with ambulatory elastomeric pump (N=4), death (N=3) or change of follow-up to another hospital (N=1). Iloprost persistence at two and five years after treatment onset was 95.9% and 83.7% (Figure 1), respectively. Nine adverse events were recorded (18.4% of patients): headache was reported in four patients, hypotension in three patients, tachycardia in one patient and generalised erythroderma in one patient.
Conclusion: SSc patients achieved clinical improvement with a good tolerability profile, leading to a high drug persistence rate. Side effects were managed by adapting the infusion rate. Our data support that monthly single iloprostinfusions can be effective, safely administered and adjusted according to the drug tolerance.
To cite this abstract in AMA style:
Martins P, Dourado E, Fonseca J, Romão V, Resende C. Safety and Persistence of Monthly Intravenous Iloprost in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/safety-and-persistence-of-monthly-intravenous-iloprost-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-persistence-of-monthly-intravenous-iloprost-in-systemic-sclerosis/