Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate the long-term safety and efficacy of upadacitinib (UPA), an oral JAK inhibitor, through week 204 in patients with RA from the long-term extension (LTE) of the SELECT-CHOICE study.
Methods: In SELECT-CHOICE (period 1: 24-week, phase 3, double-blind), RA patients refractory to biologic DMARDs were randomized to either upadacitinib 15 mg (UPA15) once daily or intravenous abatacept (ABA).1 In period 2, open-label LTE patients initially randomized to ABA were switched to UPA15 at week 24, while patients initially randomized to UPA15 continued to receive UPA15 for up to 4 years. Efficacy endpoints, including patient-reported outcomes (PROs) through week 204, were analyzed using as observed (AO) and NRI for binary endpoints or descriptive statistics based on AO and mixed-effect model repeated measures (MMRM) for continuous endpoints. Treatment-emergent adverse events (TEAEs) are summarized through week 204.
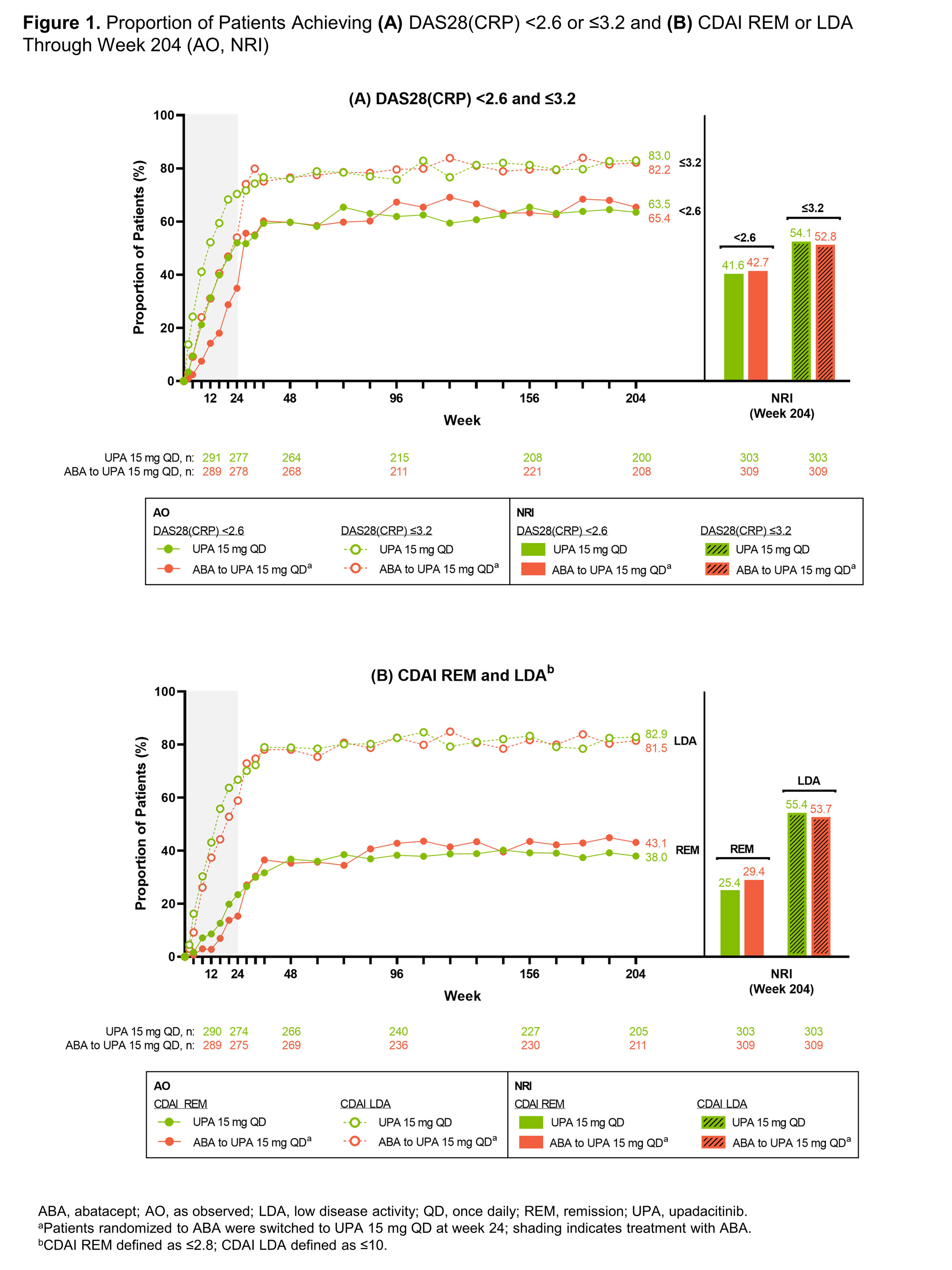
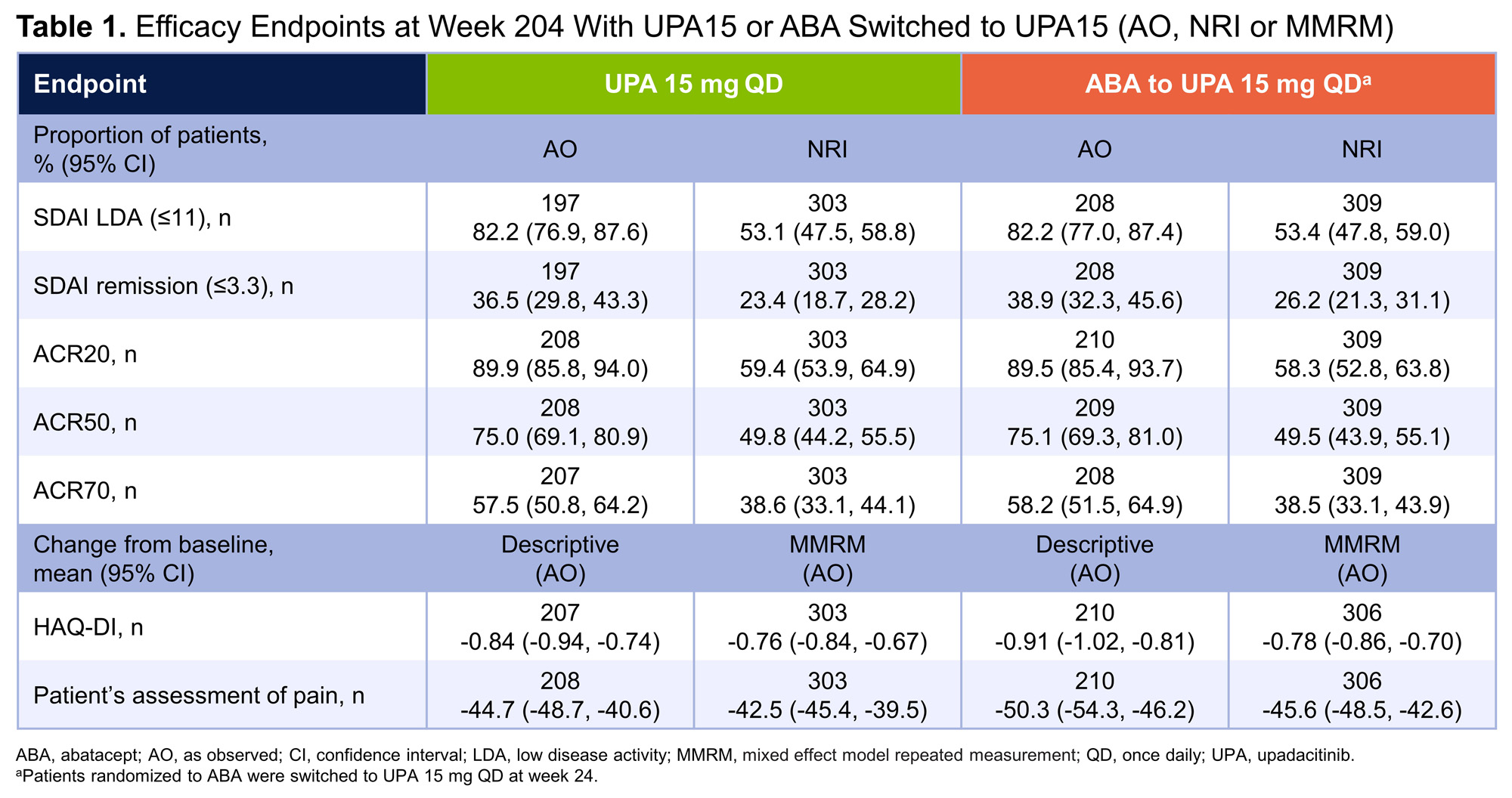
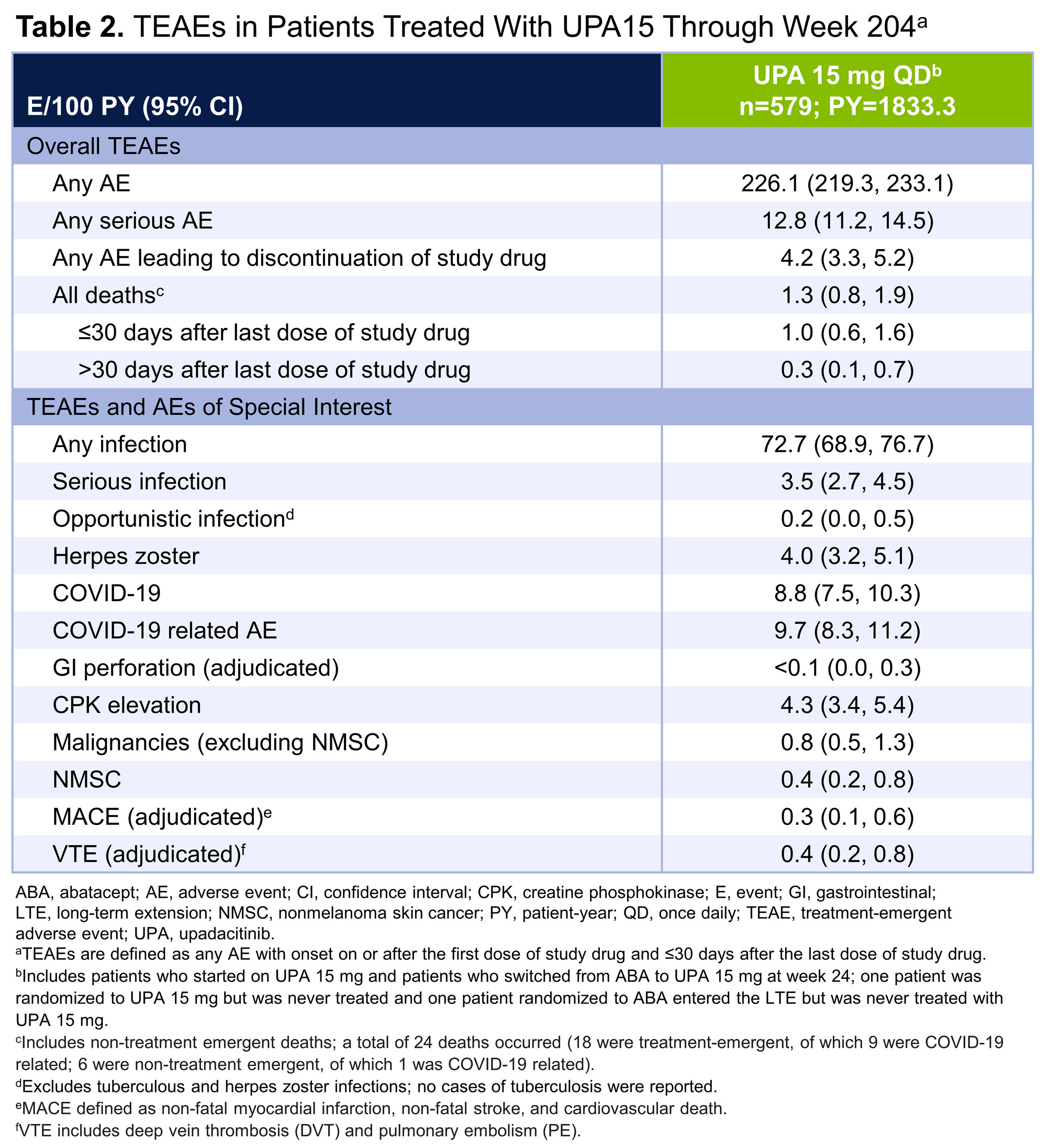
Results: In total, 304 patients were randomized to UPA15; 278 (91.4%) patients completed week 24 and 277 (91.1%) continued to the LTE. Of the 309 patients randomized to ABA, 277 (89.6%) patients completed week 24 and entered the LTE. Of the patients who entered the LTE on UPA15 (n=547), 151 (27.6%) patients discontinued UPA15 treatment, with the most common reasons being adverse event (9.1%), withdrawal of consent (5.9%), and other reason(s) (7.3%). Of patients on continuous UPA15, a high proportion achieved DAS28(CRP) < 2.6 or ≤3.2, which was maintained or further improved through week 204 (< 2.6: 63.5%; ≤3.2: 83.0%) (AO) (Figure 1A). Greater than one-third of patients achieved CDAI remission (38.0%) and over 80% achieved LDA (82.9%) at week 204 (AO) (Figure 1B); similarly, 36.5% of patients achieved SDAI remission and 82.2% achieved LDA (AO) (Table 1). At week 204, 90%/75%/58% of patients achieved ACR20/50/70 responses (AO). Boolean remission was achieved at week 204 by 26.7% (95% CI: 20.7, 32.7) of patients (AO); more conservative estimates using NRI showed similar results (19.1% [14.7, 23.6]). Mean change from baseline in HAQ-DI was -0.84 and the patient’s assessment of pain was -44.7 at week 204 (AO). Across all efficacy endpoints, similar results were observed in patients who switched from ABA to UPA15, compared to those who continued UPA15. Patients with an inadequate response to ≥1 prior anti-TNF (UPA15: n=263; ABA to UPA15: n=273) showed similar responses to the overall population (data not shown). No new safety risks were identified with long-term exposure to UPA15 in patients with RA through week 204 (Table 2). A total of 24 deaths occurred; 18 deaths were treatment-emergent, of which 9 were related to COVID-19.
Conclusion: Efficacy responses with UPA15 were maintained over time, including DAS28(CRP) < 2.6 and ≤3.2, CDAI and SDAI remission and LDA, ACR20/50/70 responses, and PRO outcomes.1 The safety profile of UPA through week 204 is consistent with previous findings1 and the broader RA clinical program. These data further support the long-term safety and efficacy of UPA for the treatment of patients with RA.
Reference:
1. Rubbert-Roth A, et al. N Engl J Med. 2020;383:1511-21.
To cite this abstract in AMA style:
Rubbert-Roth A, Kato K, Haraoui B, Rischmueller M, Liu Y, Khan N, Camp H, Xavier R. Safety and Efficacy of Upadacitinib in Patients with Rheumatoid Arthritis Refractory to Biologic DMARDs: Results Through Week 204 from the SELECT-CHOICE Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-upadacitinib-in-patients-with-rheumatoid-arthritis-refractory-to-biologic-dmards-results-through-week-204-from-the-select-choice-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-upadacitinib-in-patients-with-rheumatoid-arthritis-refractory-to-biologic-dmards-results-through-week-204-from-the-select-choice-study/