Session Information
Date: Monday, November 18, 2024
Title: Abstracts: SLE – Treatment II: Non-Cellular Lupus Therapeutics
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
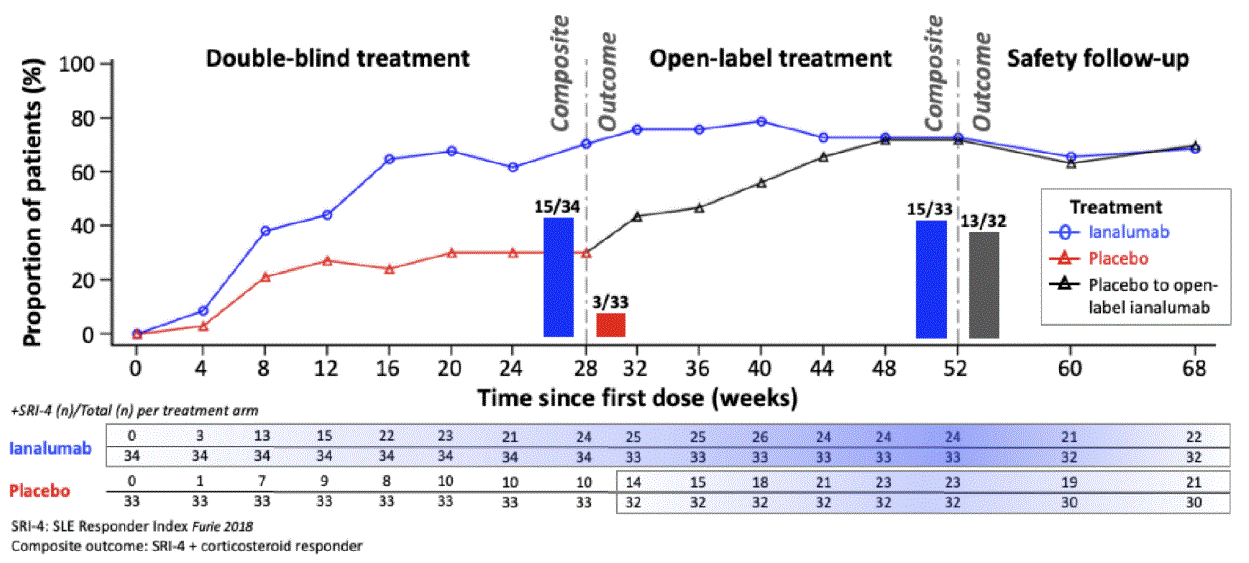
Background/Purpose: Ianalumab (VAY736) is an afucosylated, fully human IgG1 monoclonal antibody with a dual mechanism of action of enhanced B-cell depletion through antibody-dependent cellular cytotoxicity and B cell–activating factor receptor blockade. Our purpose is to expand upon previously published results from the phase 2 study with longer term (68-week [W]) results from open-label (OL) ianalumab treatment.
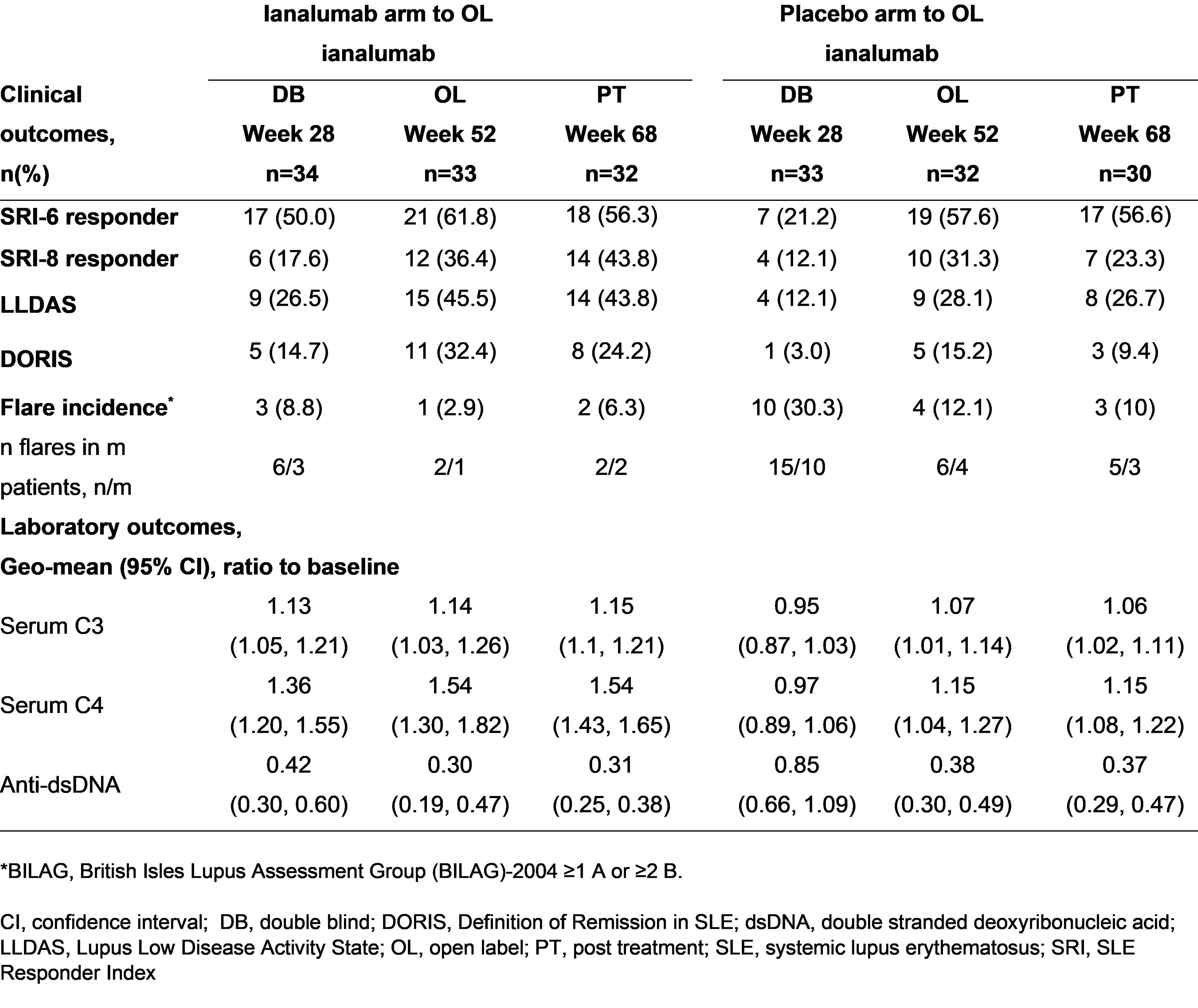
Methods: This multi-center, randomized, parallel-group trial included 3 study periods: 1) 28W blinded, placebo-controlled, 2) 24W OL, and 3) post-treatment follow up. We report here 68W results. Patients were randomized (1:1) to either subcutaneous ianalumab 300 mg or placebo every 4 weeks (q4w) and switched at W28 to OL ianalumab through W48. Patients with anti-nuclear antibodies ≥1:80, ≥4 of 11 ACR 1997 SLE classification criteria, SLEDAI-2K score ≥6, and BILAG -2004 ≥1 A or ≥2 B were included. Outcomes were measured at baseline, q4w to W52, and then W60 and W68. The primary W28 outcome was the composite endpoint of patients who achieved both SLE Responder Index (SRI-4) and met corticosteroid (CS) responder criteria (tapering prednisone ≤5 mg/d or ≤baseline dose, whichever was lower, by W16 and kept within that range to W28). W52 composite responders met CS responder criteria from W40 to W52. Other outcomes included SRI-4, SRI-6, SRI-8, incidence BILAG-2004 flares (BILAG-2004 ≥1 A or ≥2 B scores), proportion of patients achieving Lupus Low Disease Activity State (LLDAS) and Definition of Remission in SLE (DORIS), safety/tolerability, and laboratory markers for B cell counts and immune activation.
Results: Overall, 67 patients enrolled from December 19, 2018 to January 31, 2022 with median age 42 years and 39 years for ianalumab and placebo arms, respectively. Baseline median (range) values for ianalumab and placebo arms, respectively, were: SLEDAI-2K scores 11 (6-32) and 10 (4-21), and prednisone dose/day 10.0 mg (0-30.0) and 10.0 mg (0-27.5). The proportion of patients treated with ianalumab or placebo achieving composite endpoint criteria for SRI-4 + CS responder at W28 was 44.1% (n/N=15/34) vs 9.1% (n/N=3/33), respectively, and at W52 for patients switching from active treatment to OL ianalumab or switching from placebo to ianalumab was 45.5% (n/N=15/33) vs 40.6% (n/N=13/32; Figure 1). Longer duration of q4w ianalumab exposure at W52 vs W28, or for placebo+24W, resulted in further improvement in outcomes (Table 1) for incidence BILAG flare, status of SRI-6, SRI-8, DORIS and LLDAS, and serum levels of complement and autoantibodies. IgG reduction from baseline (geometric mean, 95% CI) at W52 was 0.78 (0.73-0.84). Treatment responses were maintained during the safety follow up period at W68 without unexpected or new safety findings.
Conclusion: Treatment with ianalumab was well-tolerated, and data suggests longer exposure up to 1 year provides further clinical and laboratory benefits in patients with active SLE. These positive results were maintained up to W68 during the safety follow up. Ianalumab is currently being investigated in large randomized controlled phase 3 studies (NCT05639114; NCT05624749; NCT05126277) in patients with active SLE, and in those with lupus nephritis.
To cite this abstract in AMA style:
Mysler E, Ignatenko S, Gordienko A, Cortés-Hernández J, Agmon-Levin N, Narongroeknawin P, Romanowska-Prochnicka K, Shen N, Ciferská H, Kodera M, Cheng-Chung Wei J, Leszczynski P, Liang Lan J, Wojciechowski R, Tarr T, Vishneva E, Chen Y, Kaneko Y, Finzel S, Hoi A, Okada M, Koolvisoot A, Lee S, Dai L, Kaneko H, Rojkovich B, Sun L, Zotkin E, Viallard J, Paula Magallares B, Sengupta T, Sips C, Lau C, Avrameas A, J Oliver S. Safety and Efficacy of Subcutaneous Ianalumab (VAY736) for up to 68 Weeks in Patients with Systemic Lupus Erythematosus: Results from Phase 2 Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-subcutaneous-ianalumab-vay736-for-up-to-68-weeks-in-patients-with-systemic-lupus-erythematosus-results-from-phase-2-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-subcutaneous-ianalumab-vay736-for-up-to-68-weeks-in-patients-with-systemic-lupus-erythematosus-results-from-phase-2-study/