Session Information
Session Type: Late-Breaking Abstract Poster Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Olokizumab (OKZ) is a new humanized monoclonal antibody targeting IL-6 1, 2. Here we present the results of the first phase III study of OKZ in patients with Rheumatoid Arthritis (RA).
Methods: This randomized, double-blind, placebo-controlled, multicenter study in patients with moderately to severely active RA despite methotrexate (MTX) (ClinicalTrials.gov Identifier NCT02760368, CREDO1) was carried out in Russia, Belarus and Bulgaria. Patients were randomized 1:1:1 to receive SC injections of OKZ 64 mg every 2 weeks (q2w), OKZ 64 mg once every 4 weeks (q4w), or placebo (PBO) for 24 weeks with continuation of their background MTX. Starting at Week (Wk) 14, non-responders were prescribed rescue medication (sulfasalazine and/or hydroxychloroquine) in addition to study treatment. After Wk 24, subjects either rolled over into an ongoing open-label study or entered the Safety Follow-Up Period. The primary endpoint was American College of Rheumatology 20% (ACR20) response at Wk 12. Secondary endpoints included: percentage of subjects with low disease activity, defined as Disease Activity Score 28-joint count – C-reactive protein (DAS28-CRP) < 3.2 at Wk 12, improvement of physical ability from baseline to Wk 12 measured by the Health Assessment Questionnaire-Disability Index (HAQ-DI), ACR50 response at Wk 24, and percentage of subjects with Clinical Disease Activity Index (CDAI) ≤2.8 (remission) at Wk 24. Safety outcomes, including adverse events (AEs), serious adverse events (SAEs) and laboratory abnormalities (via central lab) were assessed.
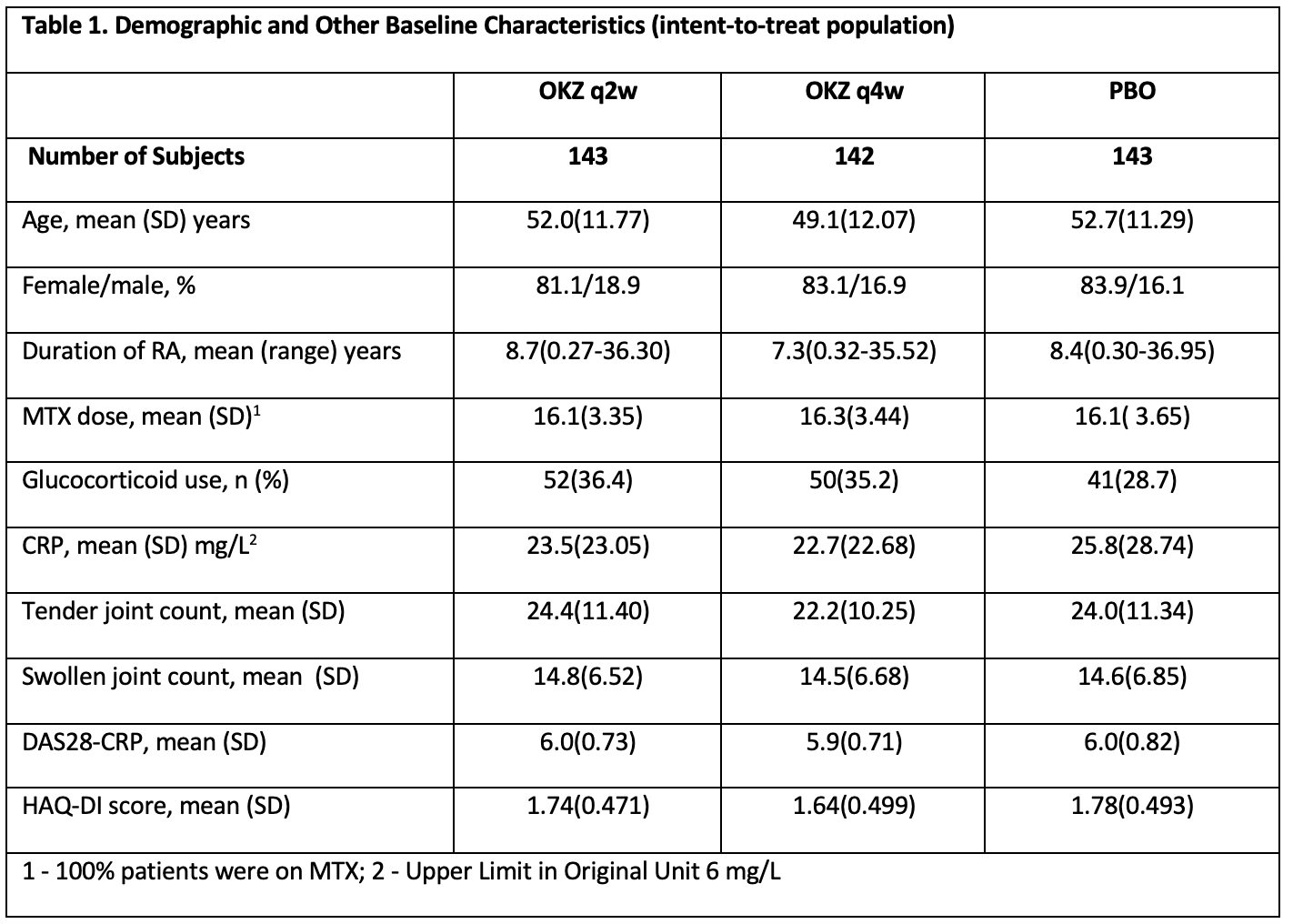
Results: A total of 428 patients were randomized to OKZ 64mg q2w (n = 143), OKZ 64mg q4w (n = 142), or PBO (n = 143). Baseline characteristics were comparable across treatment arms (Table 1).
The vast majority of patients completed 24 weeks of the study: 130 (90.9%) in q2w, 134 (94.4%) in q4w and 132 (92.3%) in PBO arms and enrolled to open label extension study CREDO4: 122 (85.3%), 127 (89.4%) and 126 (88.1%) patients, respectively.
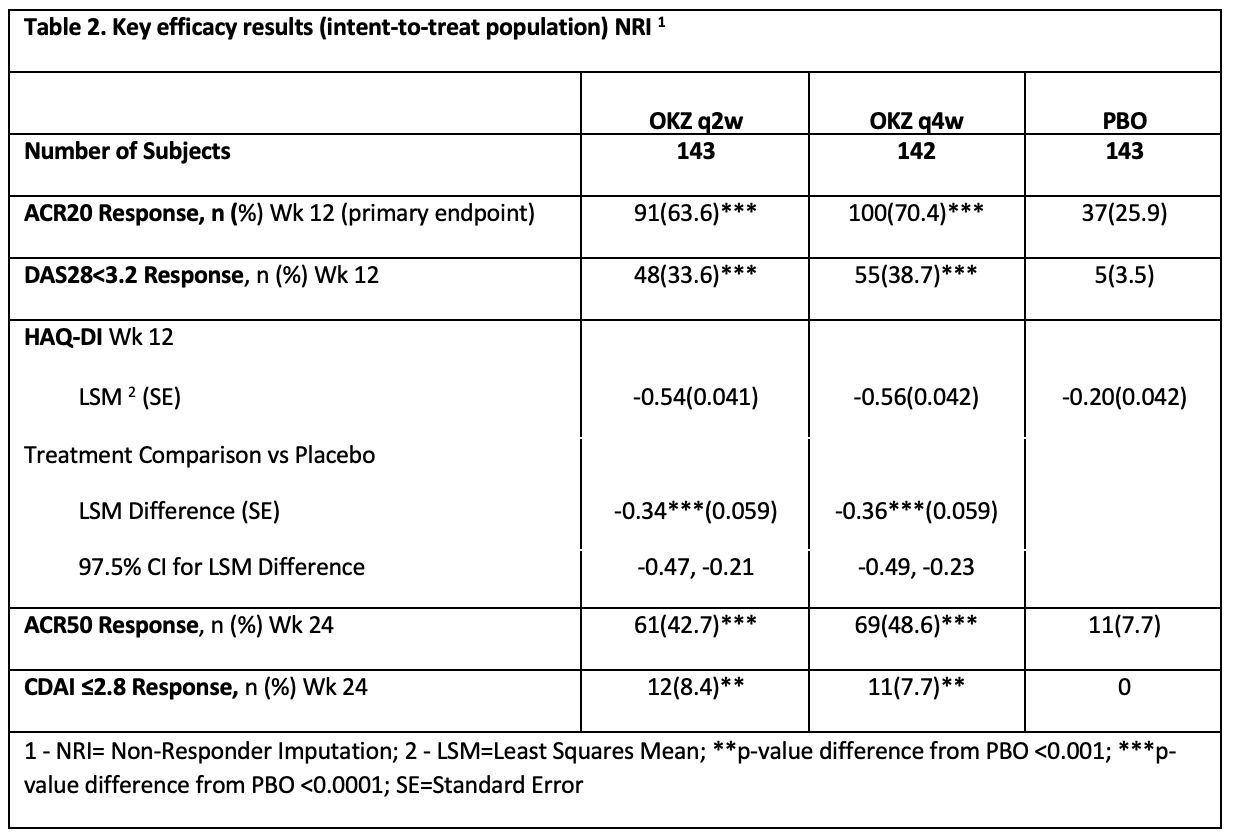
Both regimens of OKZ were significantly better than placebo in all primary and secondary endpoints (Table 2).
The key efficacy outcomes were maintained throughout the 24-week period of the study.
Overall incidence of treatment-emergent adverse events (TEAEs) was 58.0% in OKZ q2w arm; 57.0% in OKZ q4w arm and 43.7% in PBO, TEAEs leading to study treatment discontinuation were reported in 4.9%, 3.5% and 0.7% patients, respectively. There was one death due to septic shock in the OKZ q2w arm.
Incidence of treatment-emergent serious adverse events (TESAEs) were numerically higher in the OKZ groups, compared to placebo with no unexpected safety signals (Table 3).
Conclusion: In this Phase III trial, treatment with OKZ over a 24-week period was associated with significant improvements in the signs, symptoms and physical function of RA, with a safety profile consistent with Phase II data for OKZ and with the data for the agents with similar mechanism of action.
There were no discernible differences between the two regimens of OKZ in efficacy or safety outcomes.
To cite this abstract in AMA style:
Nasonov E, Fatenejad S, Korneva E, Krechikova D, Maslyansky A, Plaksina T, Stanislav M, Stoilov R, Tyabut T, Yakushin S, Zonova E, Genovese M. Safety and Efficacy of Olokizumab in a Phase III Trial of Patients with Moderately to Severely Active Rheumatoid Arthritis Inadequately Controlled by Methotrexate – CREDO1 Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-and-efficacy-of-olokizumab-in-a-phase-iii-trial-of-patients-with-moderately-to-severely-active-rheumatoid-arthritis-inadequately-controlled-by-methotrexate-credo1-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-olokizumab-in-a-phase-iii-trial-of-patients-with-moderately-to-severely-active-rheumatoid-arthritis-inadequately-controlled-by-methotrexate-credo1-study/