Session Information
Date: Tuesday, October 23, 2018
Title: Muscle Biology, Myositis and Myopathies Poster III: Treatment and Classification Criteria
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Lenabasum is a synthetic, non-immunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses. Lenabasum had acceptable safety and tolerability and improved efficacy outcomes in the double-blinded, randomized, placebo-controlled (DBPC) part A of Phase 2 trial JBT101-DM-001 (NCT02466243) in dermatomyositis (DM) subjects with refractory, skin-predominant involvement.
Objective: To provide long-term safety and efficacy data in DM subjects in study JBT101-DM-001.
Methods: Subjects who completed Part A were eligible to receive oral lenabasum 20 mg BID in an open-label extension (OLE) that assessed safety and efficacy at 4 weeks, then every 8 weeks.
Results: 20/22 (90.9%) eligible subjects received open-label lenabasum, following a mean interval of 31 weeks from end of Part A to start of OLE when they received only standard-of care. 17/20 (85.0%) subjects were on stable baseline immunosuppressive drugs. At the time of data cut-off, no subjects had discontinued from the OLE, all completed Week 22 and 17 (85%) completed ≥ Week 28. Adverse events (AEs, n = 33) occurred in 13/20 (65.0%) subjects, with 5/20 (25.0%) subjects having ≥ 1 AE (all mild) related to lenabasum. No subject had a serious or severe AEs. All subjects had AEs with maximum severity of mild (12/20, 60.0%) or moderate (1/20, 5.0%). The only AE that occurred in more than 1 subject was DM flare (n = 2, 10%); the last recorded CDASI activity score prior to flare was 14 points lower than baseline in 1 subject and 5 points higher than baseline in 1 subject. Mild dizziness occurred in 1 (5.0%) subject.
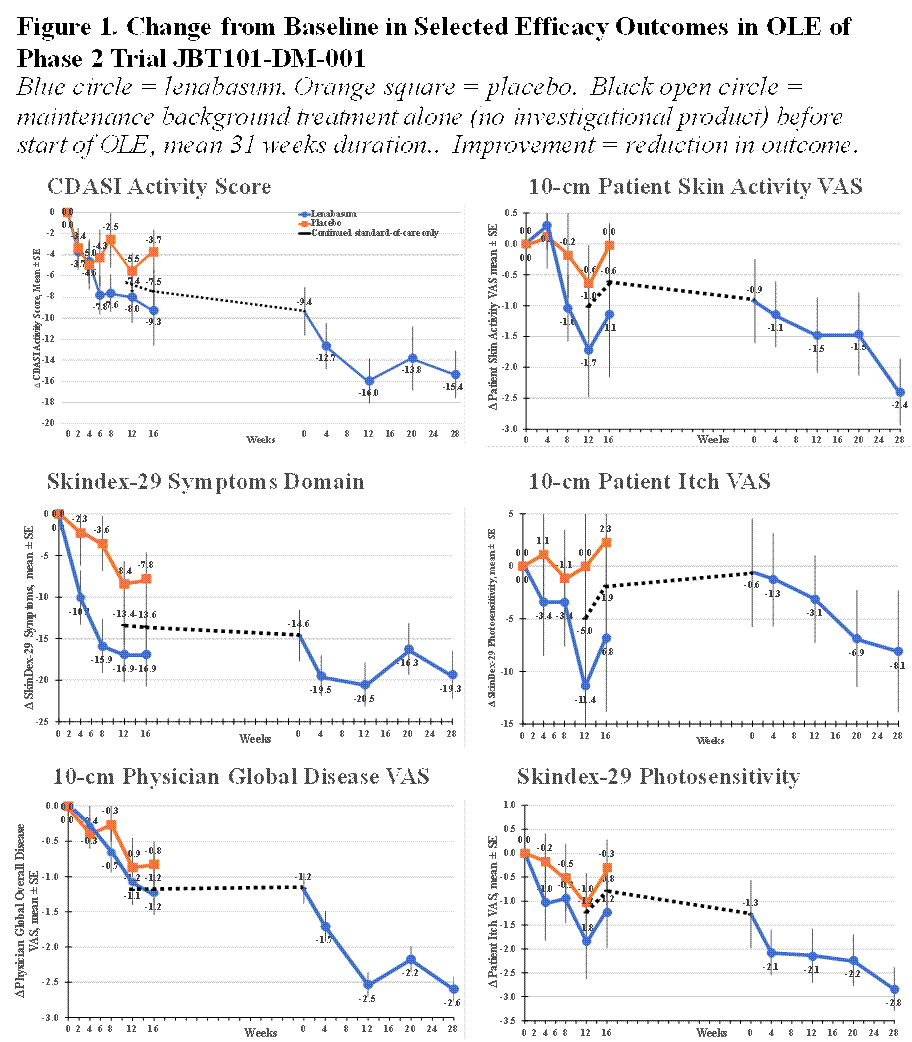
Improvement was seen in multiple physician- and patient-reported efficacy outcomes including: CDASI activity score; physician VAS assessments of skin activity and extra-muscular disease; physician Likert assessments of global disease, skin disease, and extra-muscular disease; and patient VAS assessments of overall disease, skin disease, itch, pain, and several SkinDEX-29 and PROMIS-29 domain scores. Examples in shown in Figure 1. Mean (SD) changes at Week 28 from study start were: CDASI activity score = –15.4 (9.24) points, with 14/17 (82.3%) subjects achieving ≥ 10-point improvement and 8/17 (47.1%) subjects achieving low disease activity with CDASI ≤ 14; 10-cm Physician Overall Disease VAS = -2.6 (1.90) points, with 14/17 (82.3%) subjects achieving ≥ 1 point and 20% improvement.
Conclusion: Lenabasum continues to have a favorable safety and tolerability profile in the OLE of the Phase 2 trial JBT101-DM-001 with no severe or serious AEs or study discontinuations related to lenabasum. The CDASI activity score and multiple other physician and patient-reported outcomes improved, although limitations of attributing efficacy to lenabasum in the setting of open-label dosing is acknowledged. These data support further testing of lenabasum for the treatment of DM.
To cite this abstract in AMA style:
Werth VP, Pearson D, Okawa J, Feng R, Concha J, Patel B, Hejazi E, Cornwall C, Constantine S, White B. Safety and Efficacy of Lenabasum in Refractory Skin-Predominant Dermatomyositis Subjects Treated on an Open-Label Extension of Trial JBT101-DM-001 [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-lenabasum-in-refractory-skin-predominant-dermatomyositis-subjects-treated-on-an-open-label-extension-of-trial-jbt101-dm-001/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-lenabasum-in-refractory-skin-predominant-dermatomyositis-subjects-treated-on-an-open-label-extension-of-trial-jbt101-dm-001/