Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Lupus Erythematosus – Treatment I (0801–0806)
Session Type: Abstract Session
Session Time: 2:00PM-2:15PM
Background/Purpose: Hydroxychloroquine (HCQ) is a foundational therapy in systemic lupus erythematosus (SLE) treatment, as it prolongs disease-free and damage-free survival. However, the optimal dose is debated due to dose-related risks for toxicity, and the efficacy of lower dosing (≤5 mg/kg/day) needs to be further elucidated. Therefore, we designed a systematic review and meta-analysis to assess the impact of HCQ dose on risks of HCQ-related toxicities and adverse SLE outcomes.
Methods: A comprehensive search was performed using MeSH headings and keywords (e.g., lupus, SLE, hydroxychloroquine, toxicity, flares, death or mortality, etc.) in Medline, Embase, CINAHL and Web of Science. We included observational and interventional studies associations between HCQ dose and: i) toxicity (cardiac or eye); ii) poor SLE outcomes defined as SLE flares, acute care utilization for active SLE, disease activity, death, cardiovascular disease, kidney failure, or thrombotic events in subjects with SLE. Risk of bias was assessed using standard tools for all studies. We examined pooled hazard ratios (HR) of HCQ retinopathy (primary outcome) and pooled odds ratios (OR) of toxicity when time-to-event data was not available (secondary outcome) in high vs. low HCQ dose groups ( >5 vs. ≤5 mg/kg/day) using a random effects model. Additionally, we calculated the pooled odds of SLE flares/acute care utilization for active SLE by low vs. high HCQ dose groups. Heterogeneity was assessed using I2.
Results: Among 2246 manually reviewed studies, 190 met initial inclusion. Of these, 25 studies compared HCQ-related toxicities or poor SLE outcome by weight-based HCQ dose and were included in the meta-analysis (Figure 1). Eight studies assessed the outcome of HCQ retinopathy in SLE patients, of which two assessed HRs by weight-based dose. Among eight studies assessing outcomes of SLE flares/acute care utilization, five had available data for inclusion in pooled HRs for the 5 mg/kg weight-based dosing threshold (Table 1). The pooled HR for HCQ retinopathy associated with >5 vs ≤5 mg/kg dosing was 3.83 (95% CI 1.82-8.08), Fig. 2). At the same time, the pooled HR of SLE flare/acute care utilization for active SLE associated with ≤5 vs >5 mg/kg dosing was 1.84 (95% CI 1.18-2.87). Other outcomes of interest were each assessed by fewer than two studies that compared the 5 mg/kg weight-based dosing threshold.
Conclusion: In this systematic review and meta-analysis of HCQ dose and SLE outcomes, we found that higher HCQ dosing >5 mg/kg is associated with over three times higher risk of HCQ retinopathy but nearly two times lower risk of SLE flare/acute care utilization for active SLE. These findings indicate the need for clinicians and patients to weigh the potential harms versus benefits when determining the optimal dose of HCQ in SLE treatment.
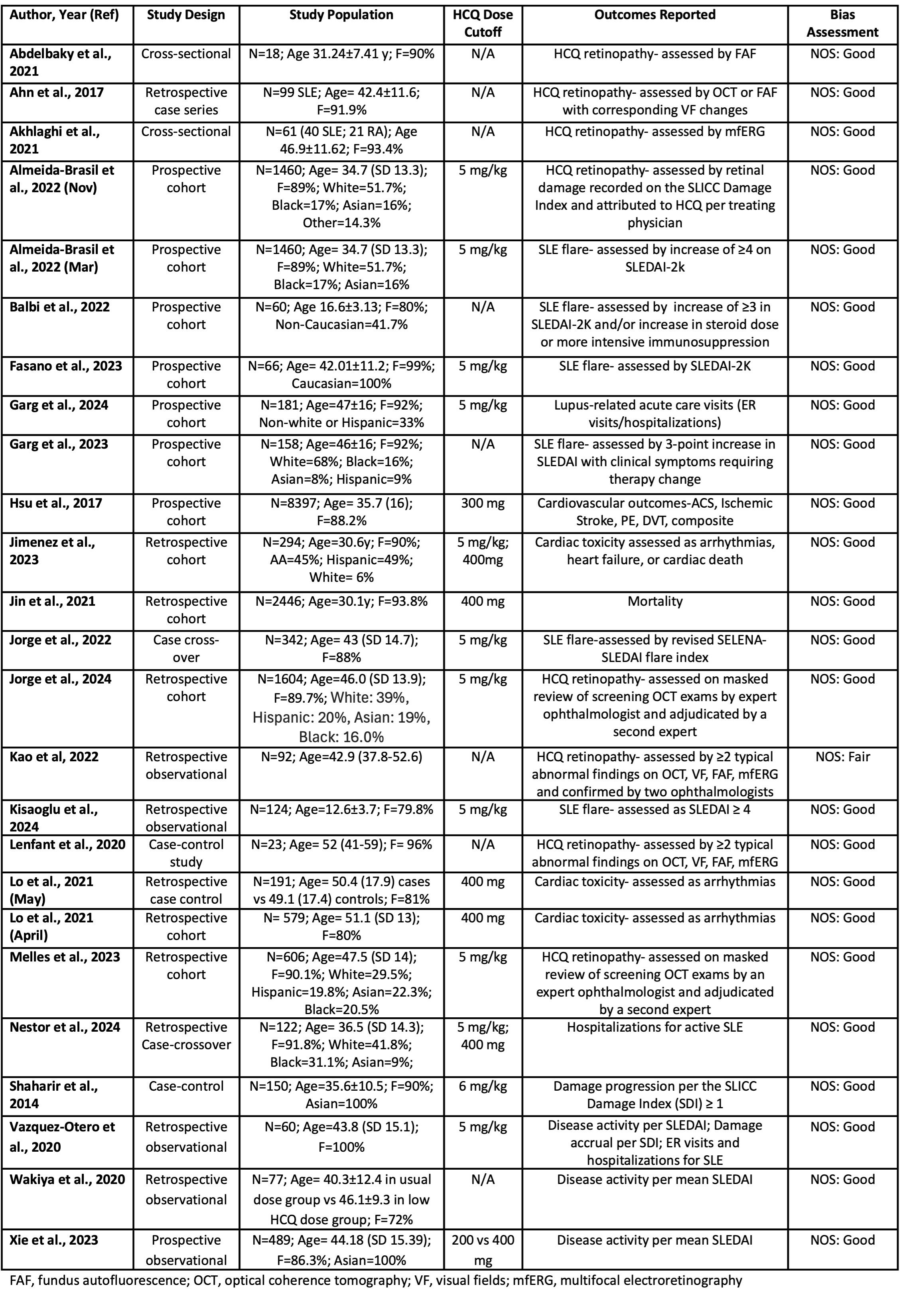 Figure 1. Literature Review and Included Studies
Figure 1. Literature Review and Included Studies
.jpg) Table 1. Included Studies Addressing the Association of Hydroxychloroquine Dose and Clinical Effectiveness, Safety
Table 1. Included Studies Addressing the Association of Hydroxychloroquine Dose and Clinical Effectiveness, Safety
.jpg) Forest Plots for A) pooled HRs for HCQ retinopathy, B) pooled ORs for SLE flares/acute care utilization for active SLE
Forest Plots for A) pooled HRs for HCQ retinopathy, B) pooled ORs for SLE flares/acute care utilization for active SLE
To cite this abstract in AMA style:
Nestor J, Qamhieh Z, Garg S, Jorge A. Safety and Efficacy of Hydroxychloroquine According to Weight-based Dose: Results of a Global Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-hydroxychloroquine-according-to-weight-based-dose-results-of-a-global-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-hydroxychloroquine-according-to-weight-based-dose-results-of-a-global-systematic-review-and-meta-analysis/
