Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science (0445–0448)
Session Type: Abstract Session
Session Time: 9:30AM-9:45AM
Background/Purpose: Denosumab (DMAb) is approved for the treatment of glucocorticoid (GC)–induced osteoporosis (GiOP). In a phase 3, international, active-controlled, double-blind, double-dummy study, treatment with DMAb was superior to treatment with risedronate (RIS) in terms of increase in BMD at both lumbar spine (LS) and total hip (TH) in GC‑treated patients with osteoporosis (Saag KG et al Arthritis Rheumatol. 2019;71:1174–1184). In this post hoc subgroup analysis, we evaluated the efficacy of DMAb vs RIS in patients with GiOP and RA.
Methods: This post hoc subgroup analysis included both GC-initiating and GC-continuing patients (≥ 7.5 mg daily prednisone or equivalent for < 3 and ≥ 3 months before screening, respectively) with GiOP and RA who were randomized 1:1 to receive DMAb (60 mg every 6 months) or RIS (5 mg once daily) for 24 months. All patients received daily calcium (≥ 1,000 mg) and vitamin D (≥ 800 IU). The primary objective of this analysis was to test if increases in LS BMD by DXA at month 12 were greater with DMAb vs RIS based on the mean difference in percent change from baseline. Secondary objectives included between-group comparisons of the mean percent change from baseline in LS, TH, and femoral neck (FN) BMD and in serum type 1 collagen C‑telopeptide (sCTX) and procollagen type 1 N-telopeptide (P1NP) at months 12 and 24; safety was assessed through the incidence of treatment-emergent adverse events (TEAEs). BMD changes were analyzed using repeated measures analysis of covariance (ANCOVA). Serum CTX and P1NP were reported as median and interquartile ranges.
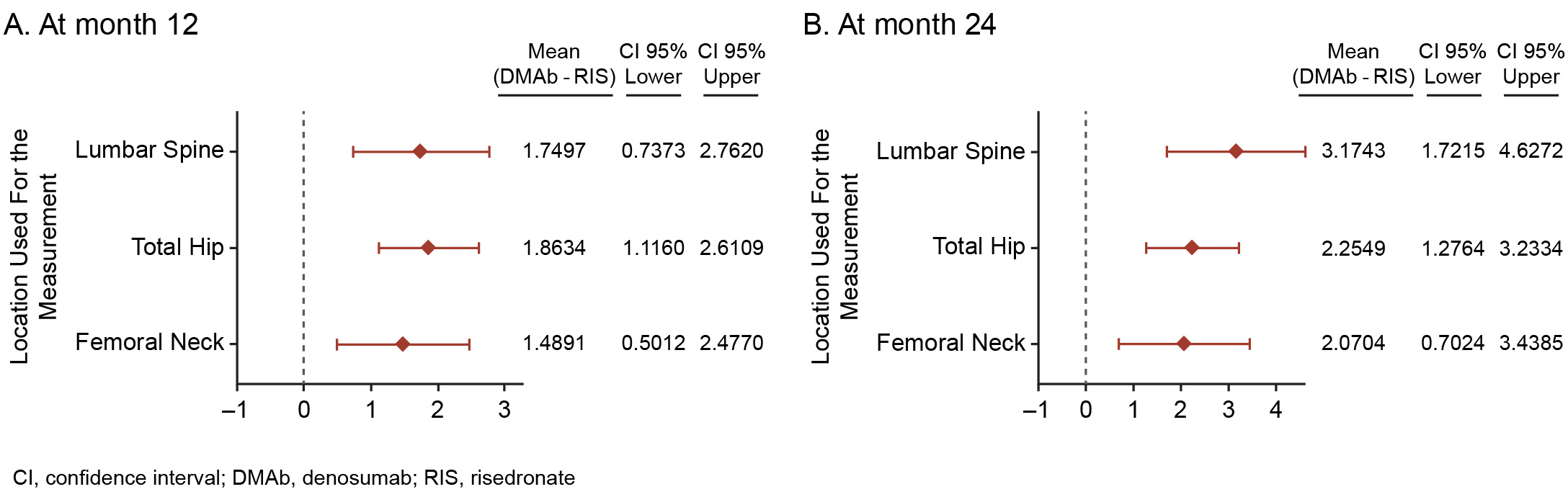
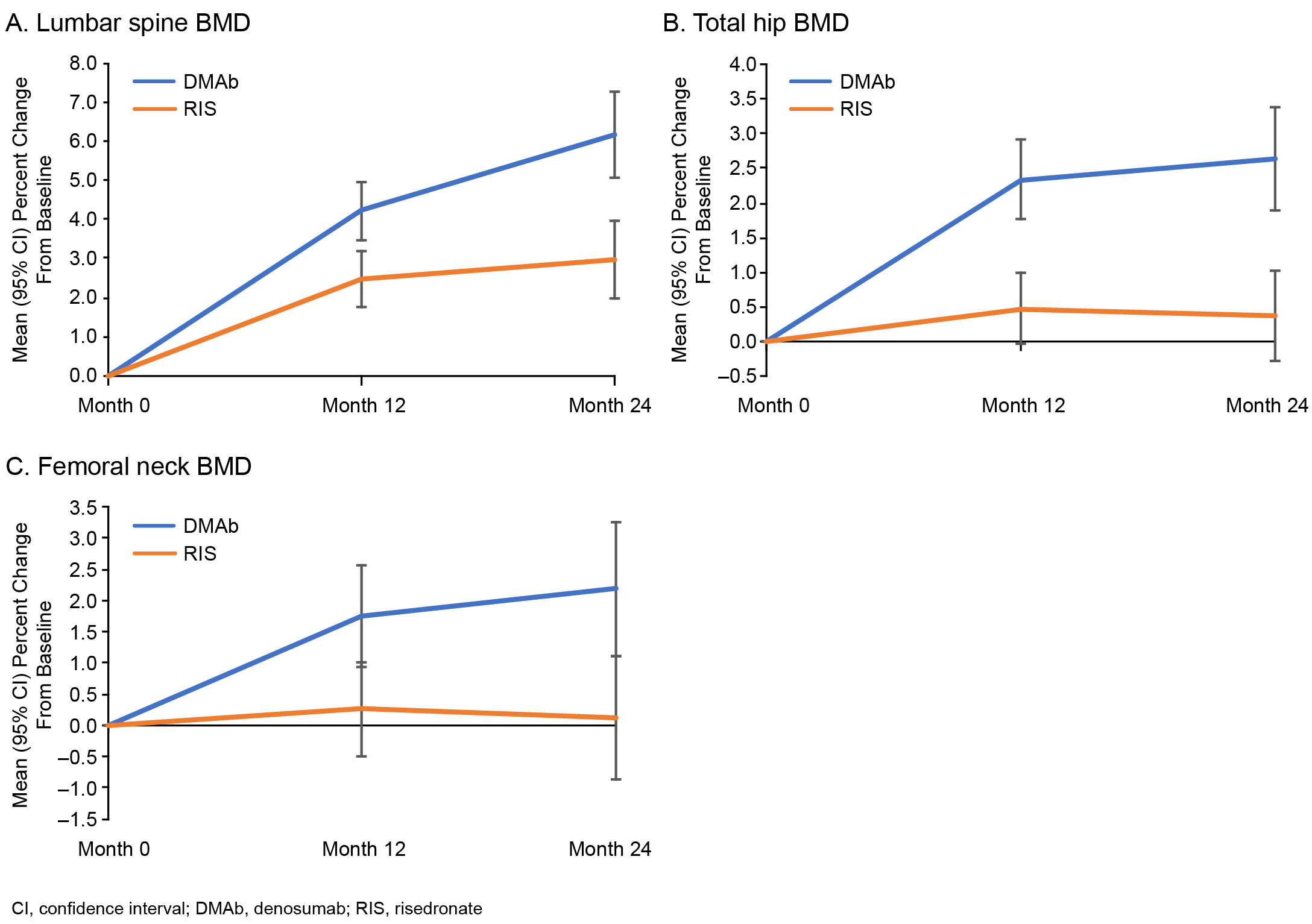
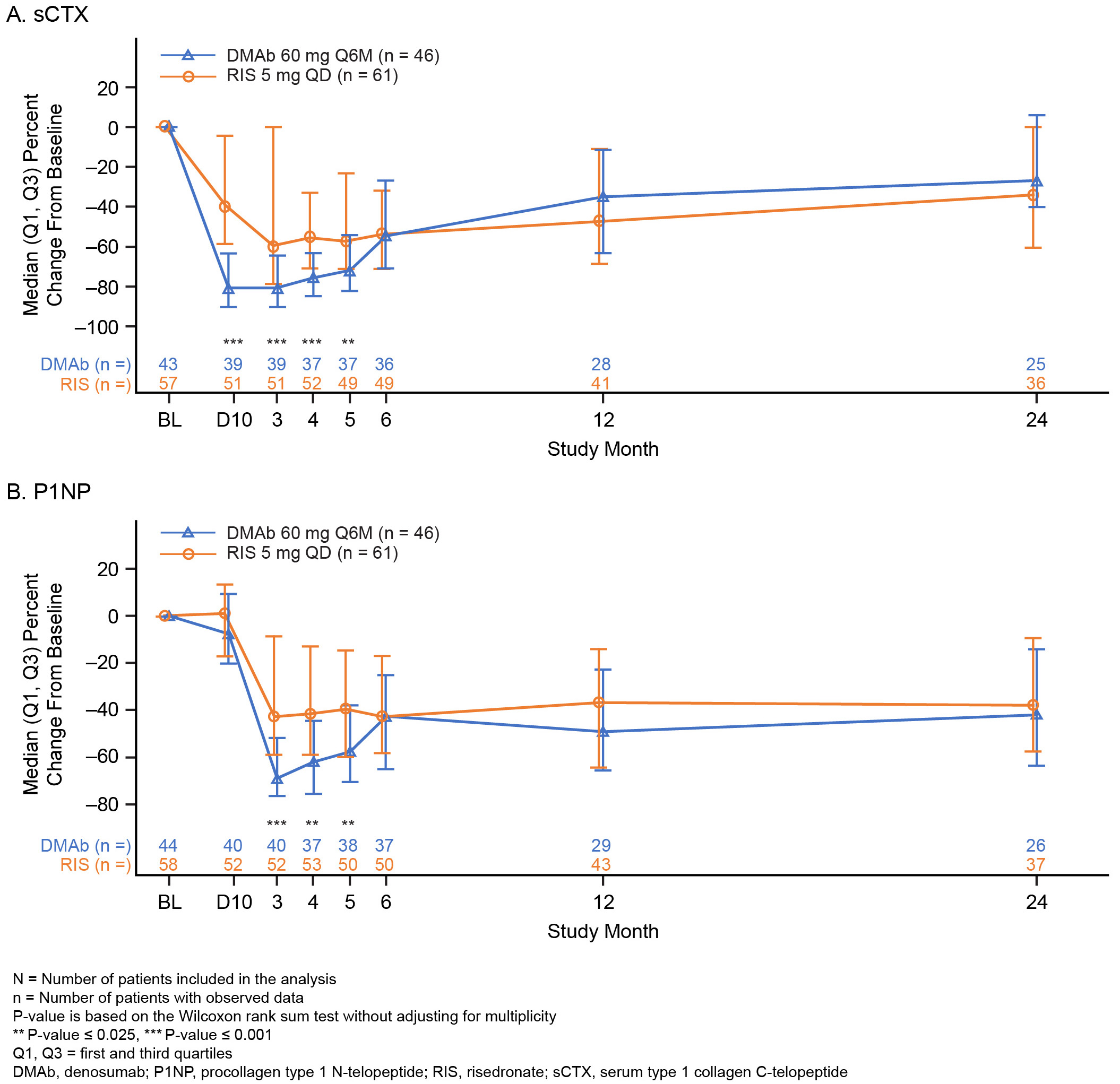
Results: A total of 300 patients (DMAb = 143; RIS = 157) were included in this analysis. Mean (SD) prednisone-equivalent doses at baseline for the DMAb and RIS groups were 10.5 (4.2) mg and 10.3 (4.6) mg, respectively. The mean difference (95% CI) in percent change from baseline in BMD between the DMAb and RIS groups was significant at LS, TH, and FN at both timepoints assessed (month 12 LS: 1.8% [0.7–2.8]; Figure 1). The mean percent change from baseline in BMD at month 24 was significant at LS, TH, and FN with DMAb and only at LS with RIS (Figure 2). A significant decrease in the sCTX and P1NP levels was observed in DMAb vs RIS patients during the first 5 months; however, the percent changes from baseline for both sCTX and P1NP were not significantly different between the DMAb and RIS groups at months 12 and 24 (Figure 3). The overall incidence of TEAEs was similar between the 2 groups (DMAb: 78.3%; RIS: 75.8%), including that of TEAEs of interest—infections (DMAb: 30.8% vs RIS: 34.4%) and cardiac disorders (DMAb: 4.9% vs RIS: 6.4%). The incidence of osteoporotic fractures was numerically lower in the DMAb (n = 9; 6.3%) vs RIS (n = 15; 9.6%) group.
Conclusion: Compared with RIS, treatment with DMAb showed significantly larger gains in BMD in patients with GiOP and RA receiving GC treatment. The overall safety profile was similar for both treatments.
To cite this abstract in AMA style:
Adachi J, Chines A, Huang S, Saag K, Lems W, Geusens P. Safety and Efficacy of Denosumab vs Risedronate in Patients with Glucocorticoid-Induced Osteoporosis and Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-denosumab-vs-risedronate-in-patients-with-glucocorticoid-induced-osteoporosis-and-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-denosumab-vs-risedronate-in-patients-with-glucocorticoid-induced-osteoporosis-and-rheumatoid-arthritis/