Session Information
Date: Sunday, November 12, 2023
Title: (0252–0282) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Retroperitoneal fibrosis (RPF) commonly affects the infrarenal abdominal aorta (irAA) and manifests with periaortic soft tissue thickening (pASTT). RPF can be either idiopathic (primary) or secondary. Secondary causes include malignancy and infection and therefore biopsy is advantageous to differentiate etiologies. However, data describing the safety and efficacy of pASTT sampling is limited. The purpose of this study was to assess the safety and efficacy of periaortic biopsies using computed tomography (CT)-guidance among a large single institution cohort of patients with RPF.
Methods: Patients undergoing CT-guided percutaneous pASTT biopsy from June 1, 1999 through September 30, 2022 were identified retrospectively. All patients were required to have radiographic evidence of soft tissue thickening/mass in direct contact with the irAA and have had CT-guided biopsy performed at Mayo Clinic. Patients with biopsies of periaortic lymph nodes or the perivascular space of the iliac vessels or inferior vena cava were excluded. Routine laboratory screening thresholds prior to biopsy included INR < 1.6 and platelet count > 50 x10^9/L). Anticoagulation/antiplatelet agents were held prior to biopsy. Charts were directly reviewed by a physician abstractor. Demographics, biopsy features, and outcomes were collected. Complications were graded based on common terminology criteria for adverse events (CTCAE) with categories 1 and 2 being minor and categories 3-5 considered major.
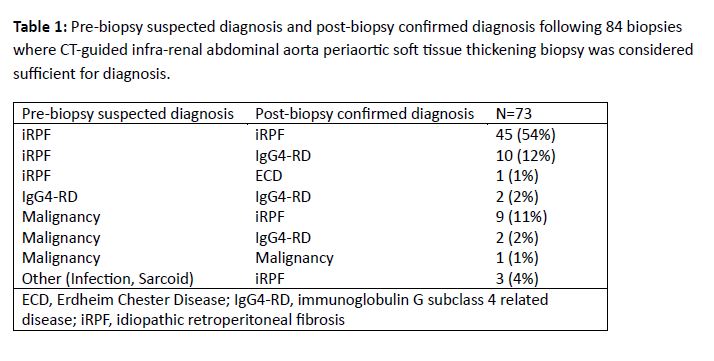
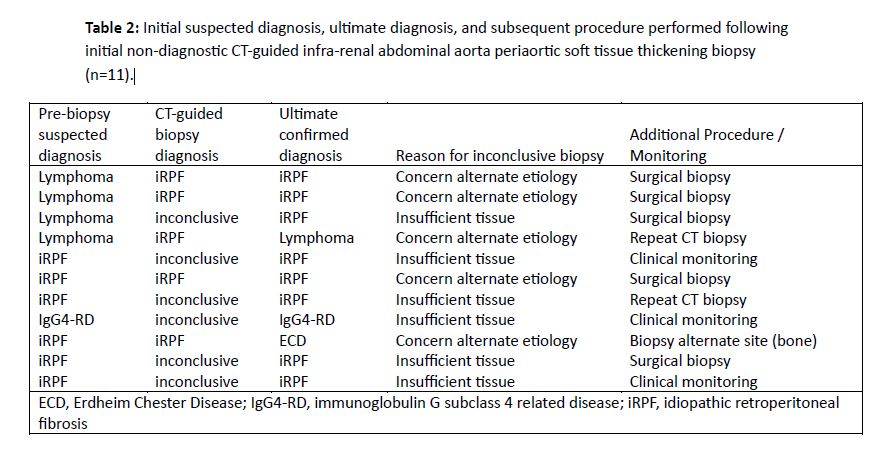
Results: A total of 83 patients (28 females, 55 males) with 84 biopsies of the pASTT at the level of the irAA were identified. Mean age at biopsy was 58 (range 31-83) years. Biopsy approach was paraspinal in 73 (87%) and anterior abdominal in 11 (13%). Mean number of passes was 5 (range 1-12). A 17/18 gauge needle size was most commonly used (67/84), followed by 19/20 gauge (15/84). One biopsy used a 15/16 gauge device and one report did not specify needle size. Local anesthesia only was used in 18 (21%) biopsies and moderate anesthesia in 61 (73%); anesthesia type was not specified in 5 (6%). Three of 84 (3.6%) biopsy events had minor bleeding at needle entry site. A single (1.2%) patient had a major bleeding complication with left rectus sheath hematoma from anterior abdominal approach requiring embolization of left inferior epigastric pseudoaneurysm. No post procedural infections were observed. Biopsy of the irAA soft tissue was able to confirm a diagnosis following 73/84 (87%) biopsies (Table 1). Among the 11 procedures where the diagnosis was not achieved by initial CT-guided irAA soft tissue biopsy, 6 were due to insufficient tissue for histologic characterization and 5 were due to concern for alternative etiology despite sufficient tissue. Among these 11 cases, 5 had subsequent open surgical biopsy, 2 had repeat CT-guided irAA soft tissue biopsy, 1 had alternative site biopsied and 3 were clinically followed (Table 2).
Conclusion: In this study, percutaneous CT-guided infra-renal pASTT biopsy was considered safe and confirmed diagnosis in 87% of cases. Ultimate diagnosis differed from pre-biopsy suspicion in 34%, highlighting benefit of this procedure.
To cite this abstract in AMA style:
Anderson C, Guarda M, Ghaffar U, Atwell T, Warrington K, Moynagh M, Koster M. Safety and Efficacy of CT-guided Percutaneous Infra-Renal Periaortic Biopsies in Suspected Retroperitoneal Fibrosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-ct-guided-percutaneous-infra-renal-periaortic-biopsies-in-suspected-retroperitoneal-fibrosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-ct-guided-percutaneous-infra-renal-periaortic-biopsies-in-suspected-retroperitoneal-fibrosis/