Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science I
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Mesenchymal stem/stromal cells are self-renewing, multi-potent stromal cells which act as modulators of immune responses. Umbilical cord-derived mesenchymal stromal cells, a classification of cells that includes umbilical cord lining stem cells (ULSC) may be associated with a younger cellular phenotype with minimal expression of HLA-DR after activation, which is expected to correlate with reduced immunogenic potential. Dermatomyositis/Polymyositis (DM/PM) is a heterogeneous disease with complex pathophysiology that may be efficiently counteracted by USLC therapy at various levels, including inhibition of plasmacytoid dendritic cell production of type-1 interferon, regulation of B cell maturation, and enhancement of T regulatory cell development. Here we have used ULSC in a phase I trial of DM/PM to evaluate the safety and tolerability of USLC in DM/PM, as well as a number of exploratory efficacy endpoints.
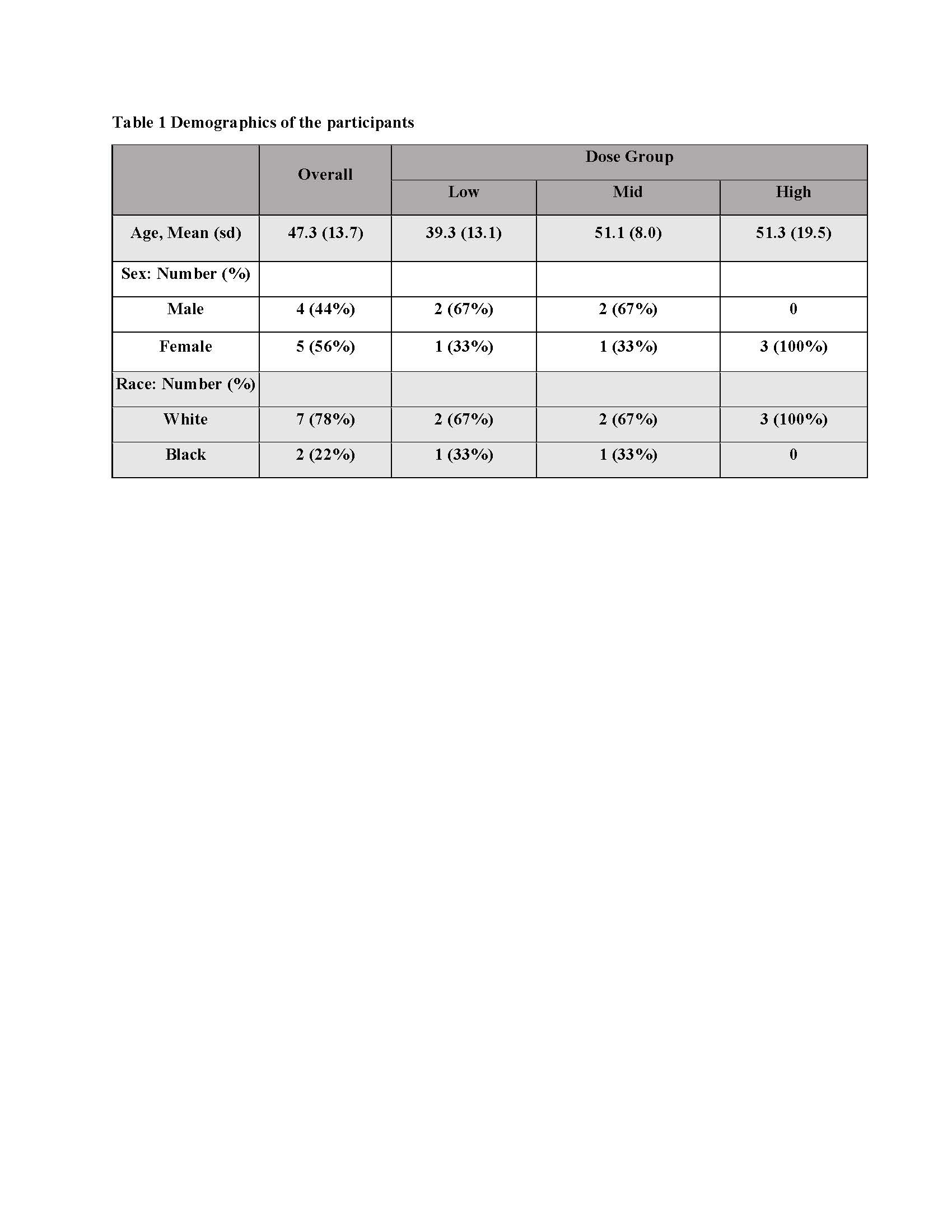
Methods: This is a phase I, first in human, open-label, non-controlled, dose-escalation trial that investigated a single intravenous infusion of ULSC in patients with PM/DM. This trial was registered prospectively with ClinicalTrials.gov (NCT04723303). Subjects had definite or probable DM or PM, with a characteristic muscle biopsy that excluded features of muscular dystrophy, metabolic myopathies, drug-induced myopathy, inclusion body myopathy, and necrotizing myopathy. Patients with DM/PM were recruited with active disease on treatment or in remission on treatment. A total of nine subjects were enrolled with 3 participants in each of 3 dose groups, consisting of an intravenous Infusion of 50, 100, or 200 million ULSC, with follow up of 12 months.
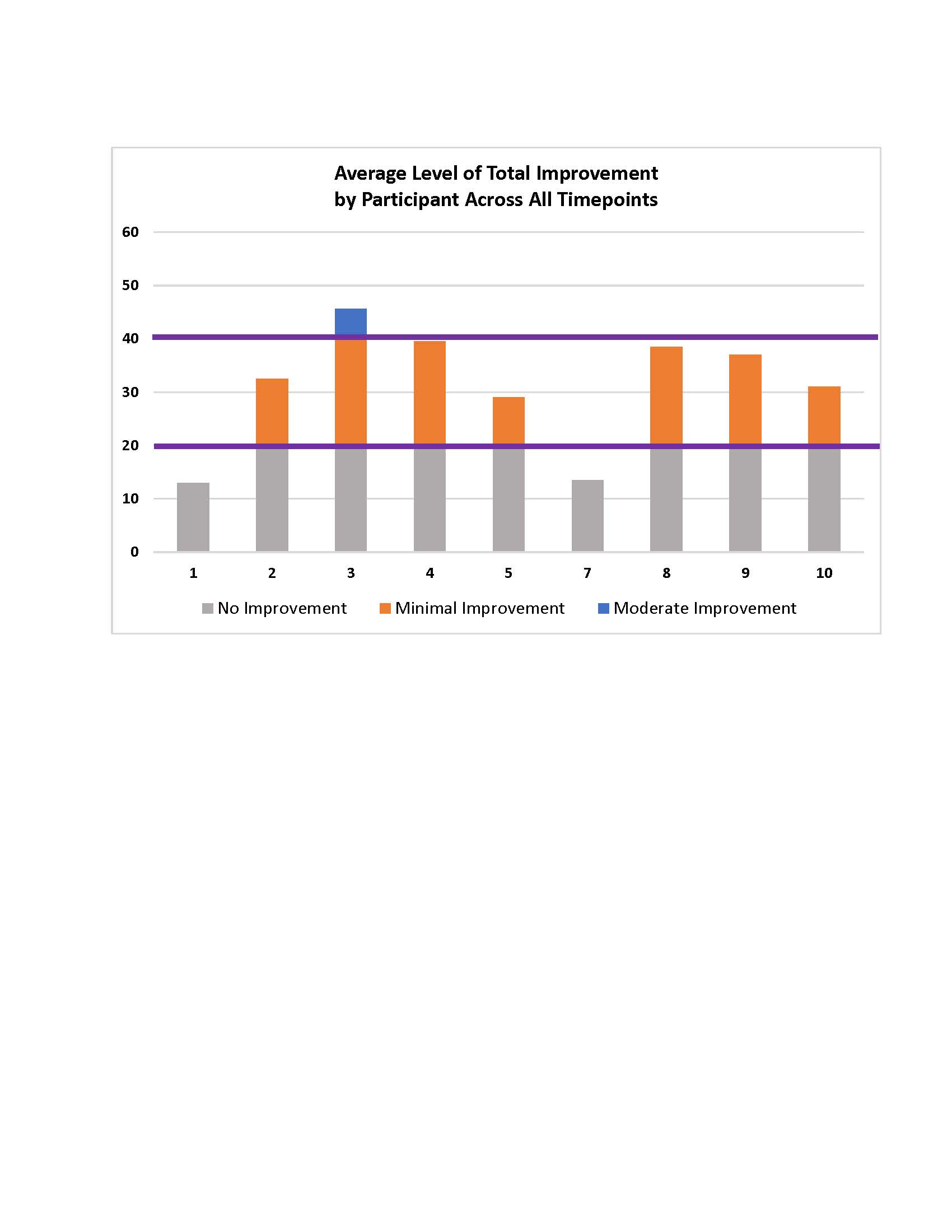
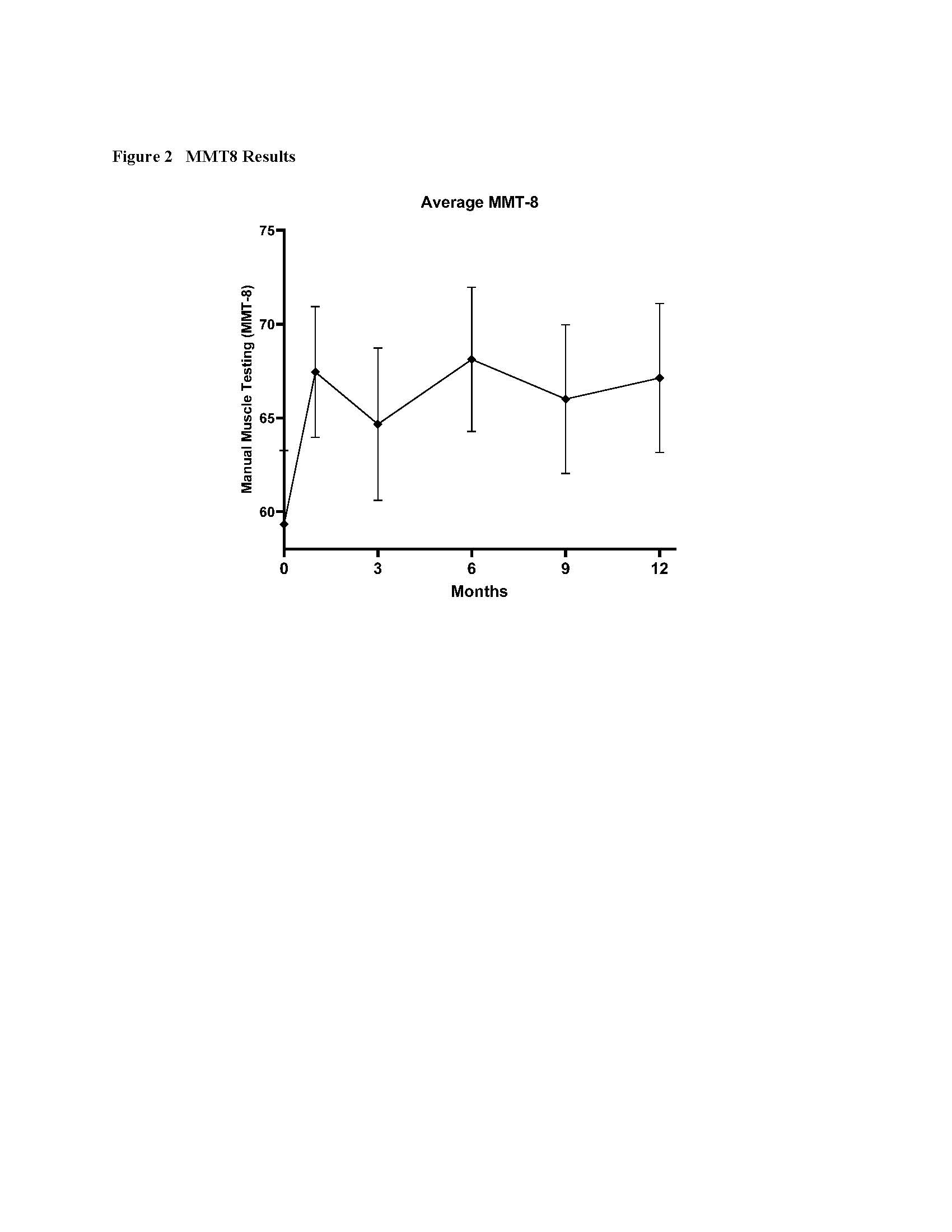
Results: Nine subjects completed the trial (Table 1). Safety and Tolerability outcomes included one Adverse Event attributed to the study product by both the Principal Investigator and the independent IDMC, a flushing reaction occurring during the infusion for the first subject. The infusion was temporarily paused, 150 mg intravenous hydrocortisone administered, and the infusion re-initiated with no recurrence. Exploratory efficacy data showed that 6 of 9 subjects met criteria for moderate improvement with total improvement score (TIS) of ≥40% – a composite outcome of six core measures of myositis activity – within 6 months of the infusion. Criteria for significant improvement, TIS of ≥20, was met by 7 of 9 subjects. The TIS averaged over 12 months is shown in Fig. 1. Manual Muscle Testing (MMT8) showed a significant improvement in strength between baseline and Month 6 of nearly 10 points on the scale, 59 ± 4 at baseline and 68 ± 4 at Month 6 (mean ± SEM), p< 0.001 (Fig. 2). Average prednisone dosage decreased by 35% at 6 months, when excluding data from one subject (a non-responder by TIS) who had a flare treated with corticosteroids at that time point.
Conclusion: Taken together, the overall human experience with the intravenous infusion of ULSC has demonstrated an absence of adverse outcomes attributable to this investigational product, and in the context of DM/PM, has demonstrated early evidence of clinical improvement in multiple participants using complementary outcome measures, in the context of progressive reduction in steroid dosing.
To cite this abstract in AMA style:
Bubb` M, Handberg E, Gonzalez R, Betancourt B, Bostick J, Long S, March K, Pepine C. Safety and Efficacy Data from a Phase I Trial of Umbilical Lining-Derived Stem Cells (ULSC) in Adult Dermatomyositis/Polymyositis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-data-from-a-phase-i-trial-of-umbilical-lining-derived-stem-cells-ulsc-in-adult-dermatomyositis-polymyositis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-data-from-a-phase-i-trial-of-umbilical-lining-derived-stem-cells-ulsc-in-adult-dermatomyositis-polymyositis/