Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
The non-interventional study ICHIBAN was set up in 2010 to evaluate the effectiveness and safety of intravenously administered Tocilizumab (TCZ IV) in patients (pts) with moderate to severe RA in Germany. Clinical data were collected during routine medical consultations including concomitant therapies, co-morbidities, therapeutic responses and adverse events. This interim analysis assessed the effectiveness and safety of long-term TCZ IV treatment with respect to patients’ age.Methods: The present interim analysis included pts with complete baseline (BL) data before 03rd December 2015 (total; n=2999). 902 pts have completed the maximal 104 week observation period (W104). Subgroups according to age have been defined as <50 years, 50-65 years, >65 years.
Results: At BL the age distribution showed the following proportions: <50 years 28.9%, 50-65 years 48.6%, and >65 years 22.5%. The mean TCZ IV treatment duration was 1.7, 1.8, and 1.7 years, respectively, for the three groups. In comparison to younger pts, the elderly (>65 years) showed longer disease duration, higher inflammatory parameters, higher disease activity, and higher co-morbidity rates at BL. Nevertheless, TCZ IV showed comparable effectiveness in all age groups. At last visit, DAS28‑BSG remission (< 2.6) was reached by 55.2%, 51.6%, and 48.8% of pts aged <50, 50-65, and >65 years, respectively. The mean reduction from BL in DAS28-BSG was 2.6, 2.7, and 2.8, respectively. Regarding co-medication, the mean glucocorticosteroid (GC) dose was reduced from 7.1 (BL) to 4.6 mg/d (last visit) and was similar in all subgroups. About 12% of pts stopped GC therapy completely, again similar in all subgroups. At baseline, the rate of TCZ monotherapy (without concomitant sDMARD) was highest in the elderly (53.2%) while combination therapy is more common in younger pts (47.1%). Despite slightly higher incidence rates of adverse events (AE) and serious adverse events (SAE) for the elderly, the rates of infections (27.2; 19.8; 19.2 per 100 patient years [PY]) and serious infections (2.9; 2.9; 3.1 per 100 PY) as well as TCZ discontinuation rates due to an AE (2.9; 2.7; 3.1 per 100 PY) did not increase with age. Gastrointestinal perforation was rarely observed (Figure 1). 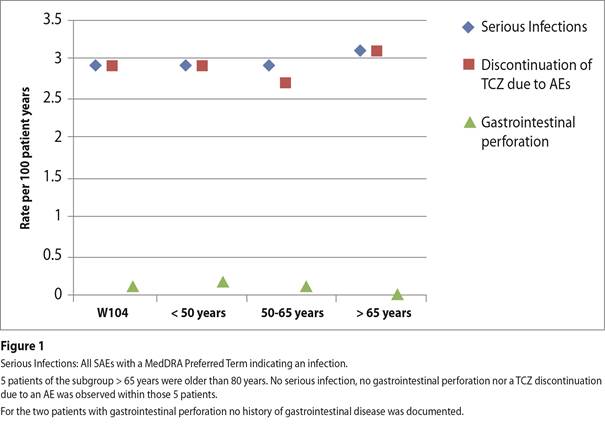
Conclusion: 902 pts were observed for 2 years. TCZ IV treatment resulted in improvements of all disease activity parameters and a reduction in concomitant GC dosing was observed. Despite higher disease burden at baseline, elderly RA pts (> 65 years) benefited to the same extent as younger pts, without increased risk of infections. Considering natural age related risks, the low infection and gastrointestinal perforation rates of elderly pts seem to be attributable to the steroid sparing effect of TCZ therapy. Safety concerns should be no reason to argue against anti‑IL‑6 therapy in elderly pts.
To cite this abstract in AMA style:
Specker C, Kaufmann J, Kellner H, Kaestner P, Volberg C, Braunewell V, Aringer M, Sieburg M, Meier L, Hofmann M, Flacke JP, Tony HP, Fliedner G. Safe and Effective Tocilizumab Therapy in Elderly Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/safe-and-effective-tocilizumab-therapy-in-elderly-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safe-and-effective-tocilizumab-therapy-in-elderly-patients-with-rheumatoid-arthritis/
