Session Information
Date: Sunday, November 13, 2022
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: It is known that type 1 IFN and proinflammatory cytokines are associated with the pathogenesis of systemic lupus erythematosus (SLE). Recently, the S100 protein, a damage-associated molecular pattern (DAMP) factor, was reported to associate with type 1 IFN and SLE disease activity. We reported that HCQ modulated elevated expression of S100 proteins in SLE (1). HCQ is a standard treatment for SLE, however, which cytokines or biomarkers that serve as efficacy indicators of HCQ have not been clarified. The study aims to determine if which cytokines or S100 protein is a useful biomarker for HCQ treatment efficacy in patients with SLE.
Methods: Patients were evaluated prior to HCQ administration and every three months thereafter for a year. The Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)-SLEDAI and Lupus Low Disease Activity State (LLDAS) scales were used to measure SLE disease activity. Serum cytokines (TNF-α, IL-6, IL-8, MCP-1, IL-1ra, VEGF-A, and MIP-1a) were measured with a multiplex Luminex assay, and serum S100A8 and S100A9 levels were measured with ELISA.
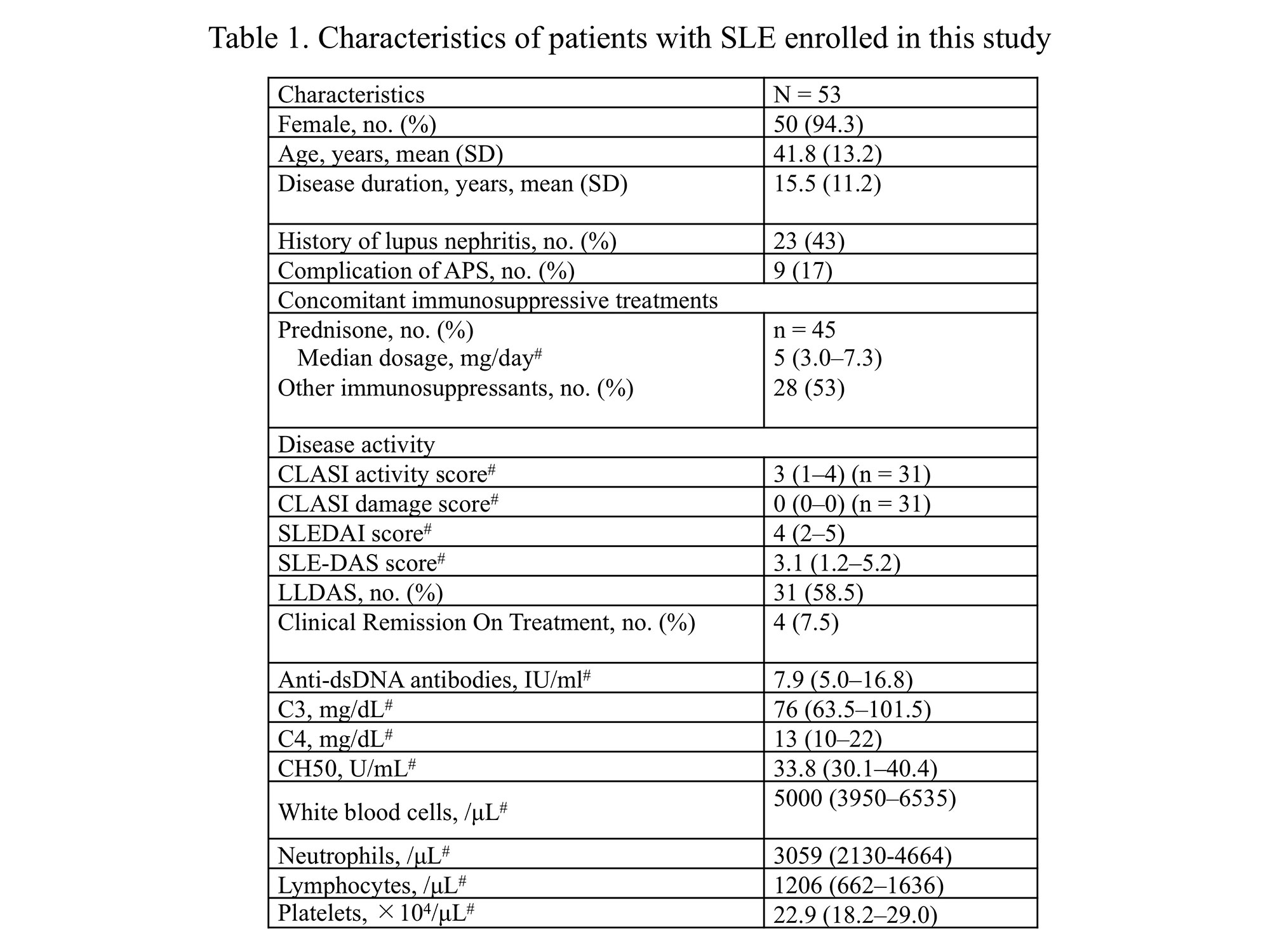
Results: Fifty-three subjects (3 males and 50 females, mean age 41.8 years) were included in this study (Table 1). No significant associations were found between baseline serum cytokine levels and serum S100A8 or S100A9 levels and disease activity indices. In addition, the relationship between baseline serum S100A8 and S100A9 levels and inflammatory cytokines was examined; however, no significant association was detected. After 3 months of HCQ treatment, SLEDAI score and anti-dsDNA antibody levels significantly improved. Out of 18 patients who did not achieve LLDAS at baseline, 14 achieved LLDAS at 12 months of HCQ treatment. As for biomarkers, the serum levels of TNF-α, IL-6, IL-8, IL-1ra, VEGF-A, MIP-1a, S100A8, and S100A9 were all significantly decreased. However, there was no association between serum inflammatory cytokines before HCQ administration and changes in SLE disease activity before and after HCQ administration.
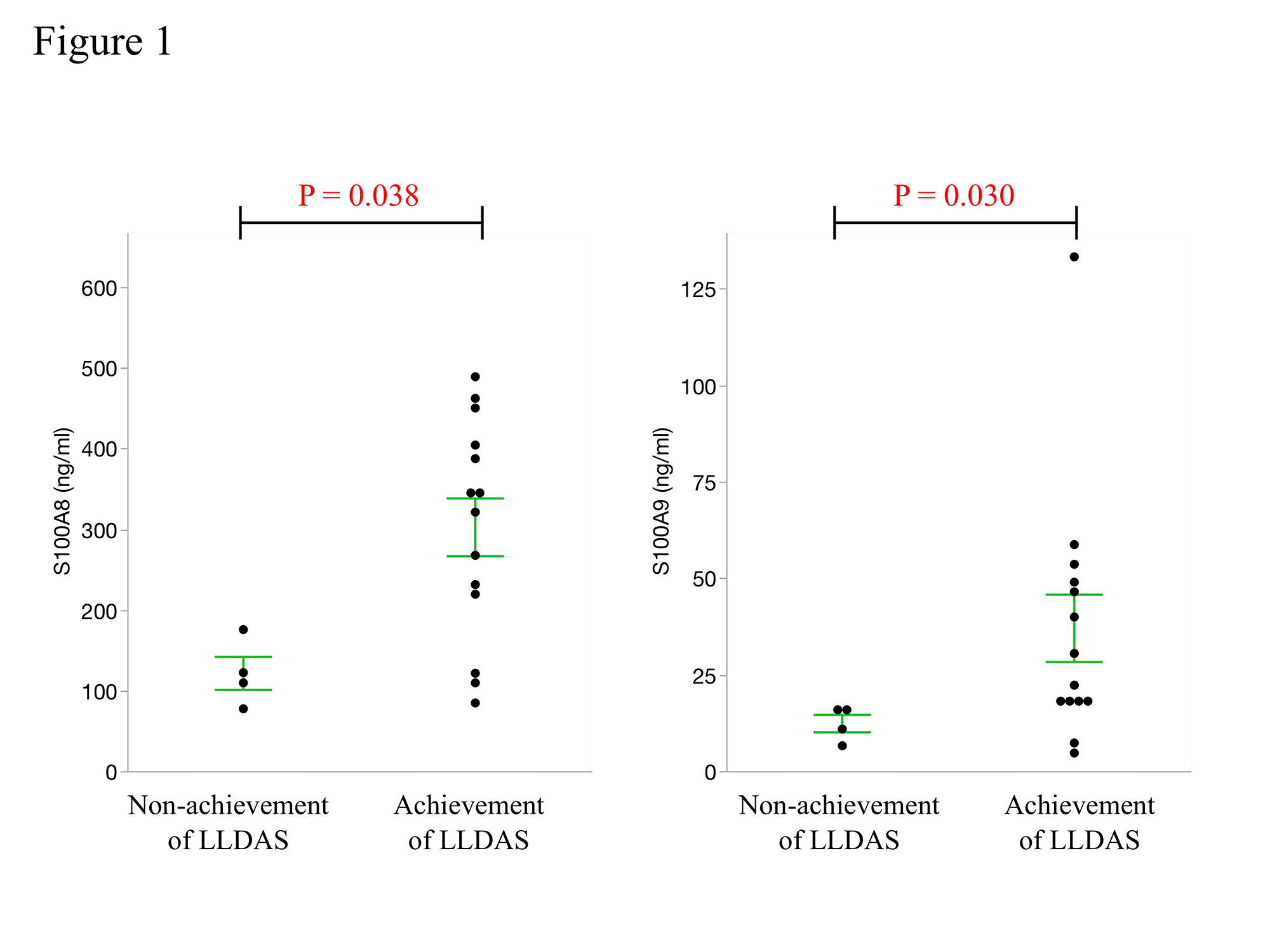
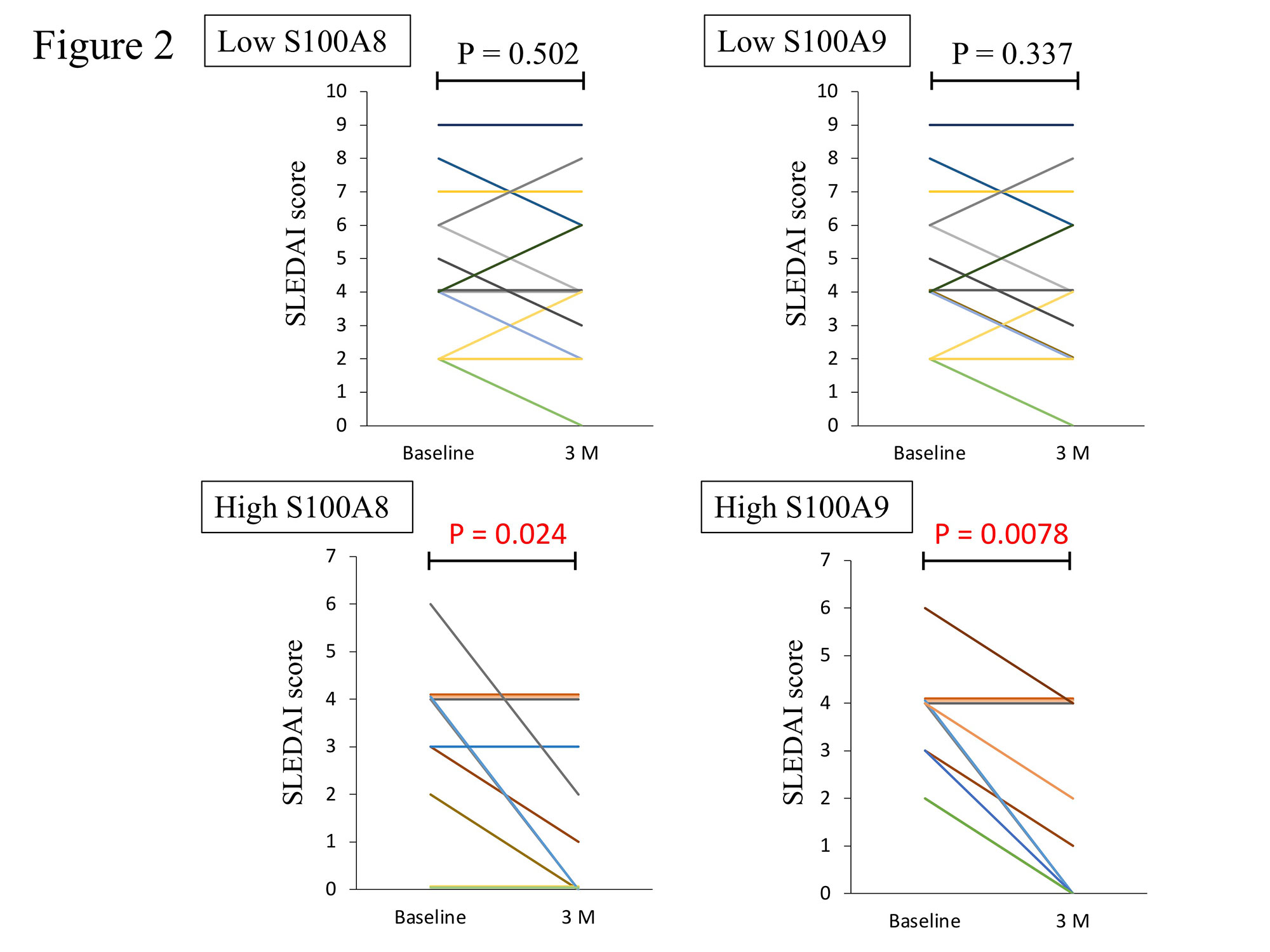
Serum S100A8 and S100A9 levels before HCQ administration were higher in patients who achieved LLDAS after HCQ administration and were significantly higher in patients who achieved LLDAS at 12 months compared to those who did not achieve LLDAS (S100A8: p = 0.038, S100A9: p = 0.030, Figure 1). SLEDAI score was significantly lower at 3 months after HCQ treatment in patients with higher S100A8 and S100A9 levels at baseline, but no significant improvement in SLEDAI score was observed in patients with lower S100A8 and S100A9 levels at baseline (Serum S100 levels were divided into high and low groups by quartile of protein level; high S100A8: p = 0.024, high S100A9: p = 0.0078 and low S100A8: p = 0.502, low S100A9: p = 0.337, Figure 2).
Conclusion: Our results suggest that measuring serum S100 protein levels can help select patients who would most benefit from HCQ treatment. IFN-α inhibitors, including HCQ administration was effective in patients with SLE with high S100A8 and S100A9 levels, suggesting that S100 serum protein level may be a useful biomarker for precision medicine approaches.
#Nonparametric distributions are represented as median (interquartile range [IQR]).
APS: anti-phospholipid antibody syndrome, LLDAS: Lupus Low Disease Activity State
Patients who achieved LLDAS by 12 months after HCQ administration had significantly higher baseline serum S100A8 and S100A9 levels. Central line represents the mean and error bars indicate standard errors. P-values were determined using the Mann–Whitney U test. A p-value of less than 0.05 was considered statistically significant.
Serum S100A8 and S100A9 levels were divided into high and low groups by quartile of protein expression level. Baseline serum S100A8 and S100A9 levels were divided into quartiles and those lower than the first quartile were defined low and those higher than the third quartile were defined high. (low S100A8; n = 13, low S100A9; n = 13, high S100A8; n = 11, high S100A9; n = 12). Changes in SLEDAI scores prior to and 3 months after HCQ administration were analyzed separately by baseline S100 protein levels. P-values were determined using the paired t test or Wilcoxon rank sum test. A p-value of less than 0.05 was considered statistically significant.
To cite this abstract in AMA style:
Wakiya R, Shimada H, Ueeda K, nakashima S, Kato M, Miyagi T, Ushio Y, Sugihara K, Mino R, mizusaki m, Chujo K, Kameda T, Dobashi H. S100 Serum Protein Expression Is a Useful Indicator of HCQ Treatment Efficacy in SLE [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/s100-serum-protein-expression-is-a-useful-indicator-of-hcq-treatment-efficacy-in-sle/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/s100-serum-protein-expression-is-a-useful-indicator-of-hcq-treatment-efficacy-in-sle/