Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is a composite tool unique to measure disease activity in ankylosing spondylitis (AS) just as Disease Activity Score 28 (DAS28) is for rheumatoid arthritis (RA). Routine Assessment of Patient Index Data 3 (RAPID3) is another composite tool that measures physical function, pain and patient global assessment in various rheumatic diseases. It correlates well with DAS28 in RA. If RAPID3 can also be used to assess disease activity in AS it may save time and costs in busy practices. Objectives of this study were to analyze the association between BASDAI and RAPID3 in patients with AS, and to obtain a cut-off value for RAPID3 which correlates with BASDAI of 4 (indicative of high disease activity).
Methods:
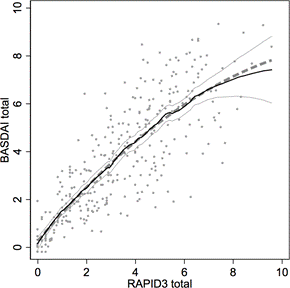
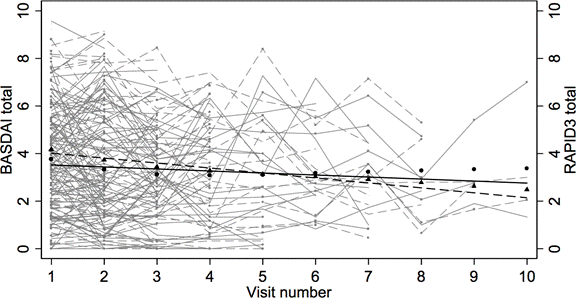
An electronic medical record search identified 157 patients with AS at our university who were followed between 2007 to 2012. Of these, 113 patients had BASDAI and RAPID3 measured at each visit (intervals ranged from 1 to 57 months). Nonparametric receiver operating characteristic (ROC) determined a cut-off of RAPID3 best correlating with BASDAI ≥ 4. RAPID3 captures both pain and function, but BASDAI measures predominantly pain without function, so multiple linear regression modeled BASDAI with RAPID3 and RAPID32 while controlling for visit number. Individual BASDAI and RAPID3 scores and summaries were plotted and compared over time.
Results:
Of 113 patients, 75 (66%) were male and 38 (34%) were female, age averaged 44.3 (SD: 13.7), disease duration averaged 8.4 years (SD 9), 77 (61%) had more than one visit, and 36 (39%) had only one. At baseline BASDAI and RAPID3 averaged 4.17 and 3.79 respectively. BASDAI was explained by RAPID3 (b = 1.218; s.e. = 0.110, p < 0.001), RAPID32 (b = -0.045; s.e. = 0.014, p = 0.002), and visit number (b = -0.141; s.e. = 0.038, p < 0.001) with adjusted R2 = 0.691. RAPID3 cut-off was 3.33 for 82.4% correct classification, 87.5% sensitivity and 78.8% specificity.
Conclusion:
RAPID3 correlates well with BASDAI, and along with pain, it provides additional information about patient function. Since it also correlates with measures of disease activity in RA and other rheumatic diseases, RAPID3 could be an attractive measure to be used in busy rheumatology practices.
Nonparametric regression of BASDAI on RAPID3 (black with 95% CI in gray) controlling for visit number suggested we model BASDAI as a quadratic function of RAPID3 (thick dashed).
Individual BASDAI (thin dash) and RAPID3 (thin solid); regressions of BASDAI (thick dash) and RAPID3 (thick solid); locally weighted estimate BASDAI (▲) and RAPID3 (■).
Disclosure:
A. Danve,
None;
K. Vakil-Gilani,
None;
A. Reddy,
None;
A. Sheffield,
None;
A. Dinno,
None;
A. A. Deodhar,
AbbVie, Merck-Sharp-Dohme, Pfizer and UCB,
5,
AbbVie, Merck-Sharp-Dohme, Pfizer and UCB,
8,
AbbVie, Amgen, Novartis, UCB,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/routine-assessment-of-patient-index-data-3-scores-correlate-well-with-bath-ankylosing-spondylitis-disease-activity-index-in-the-assessment-of-disease-activity-and-monitoring-progression-of-ankylosing/