Session Information
Date: Monday, November 11, 2019
Title: 4M114: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science (1872–1877)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
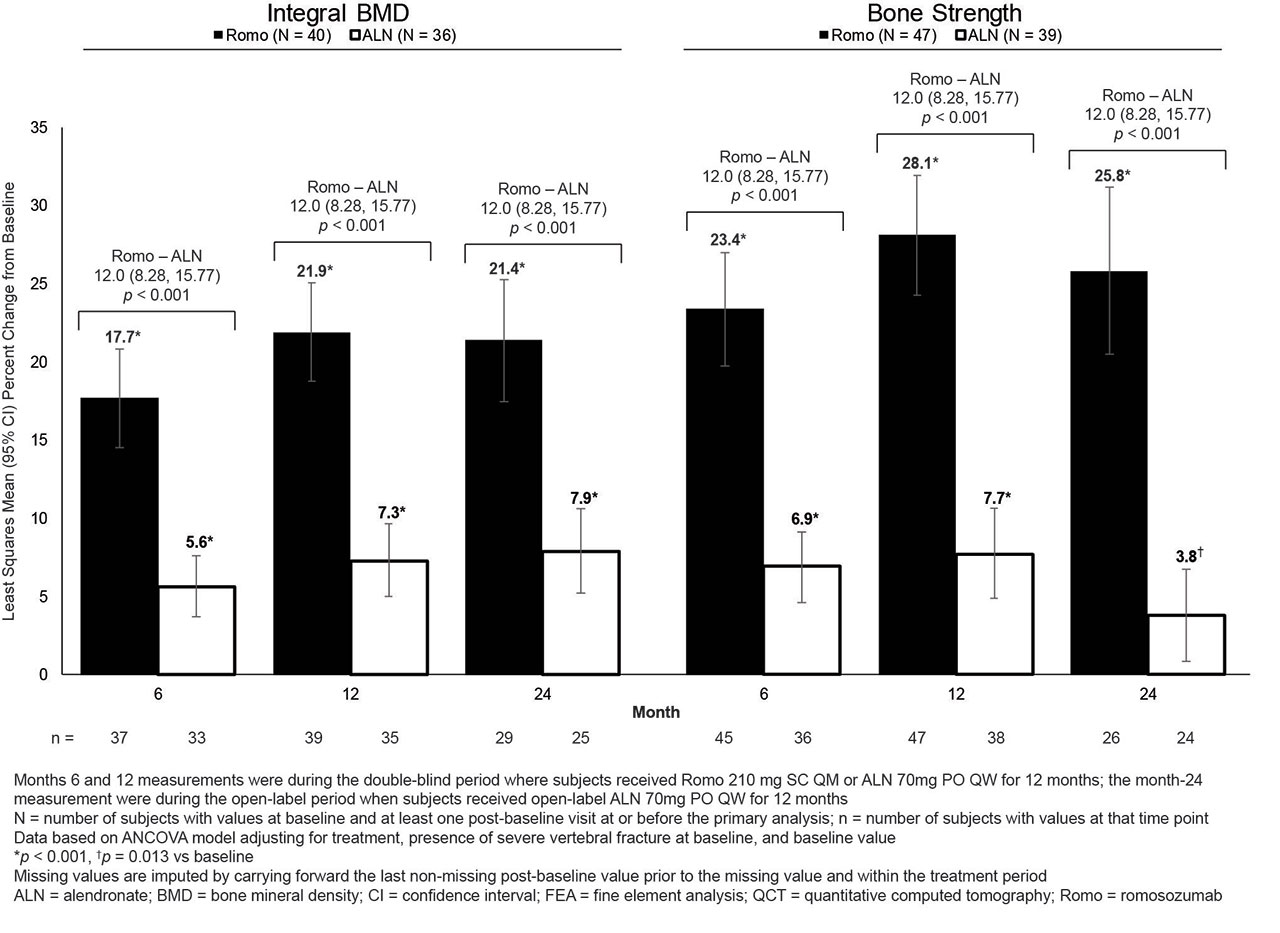
Background/Purpose: Recent evidence suggests BMD achieved during treatment is a reliable surrogate for fracture risk reduction (Bouxsein JBMR 2019). Romosozumab (Romo) is a bone-forming agent with a dual effect of increasing bone formation and decreasing bone resorption. In ARCH (NCT01631214), Romo followed by alendronate (ALN) had greater efficacy in BMD gains and fracture risk reduction vs ALN alone (Saag NEJM 2017). Here we assessed improvements in lumbar spine (LS) BMD and bone strength with Romo vs ALN treatment by quantitative computed tomography (QCT) and Finite Element Analysis (FEA).
Methods: Postmenopausal women with osteoporosis and prior vertebral or hip fracture were randomized 1:1 to receive Romo 210mg SC monthly or ALN 70mg PO weekly for 12 months, followed by open-label ALN 70mg PO weekly. In an imaging substudy, LS BMD was assessed by QCT and vertebral-estimated bone strength by FEA using QCT images obtained at baseline and months 6, 12, and 24. Correlation analyses evaluated the relationship between changes in FEA, QCT, and DXA.
Results: This post-hoc analysis included 90 subjects (49 Romo, 41 ALN) with baseline and ≥1 post-baseline QCT/FEA assessment. At baseline, mean (SD) age was 73 (7) years; DXA T-scores were –3.08 (1.09), –2.70 (0.68), and –2.84 (0.45) at the LS, total hip, and femoral neck, respectively; and 97% had prior vertebral fracture, similar to core study subjects. 76 (40 Romo, 36 ALN) subjects had QCT assessments and 86 (47 Romo, 39 ALN) had FEA assessments at baseline and ≥1 post-baseline visit. Subjects in both groups experienced significant gains in integral and trabecular BMD from baseline at all time points (except ALN in trabecular BMD; Fig and data not shown). Differences between Romo and ALN were significant at months 6, 12, and 24. QCT BMD increases were accompanied by significant increases in LS bone strength in both groups at all time points, with significantly greater increases observed with Romo than ALN. With treatment arms combined, correlation between post-baseline percent change in FEA and BMD by QCT (integral and trabecular) and DXA was similar (r=0.69–0.87, all p< 0.001).
Conclusion: Compared with ALN, Romo significantly improved LS BMD by QCT and bone strength by FEA. These effects occurred rapidly (month 6), were sustained over 12 months, were preserved upon transition to ALN through 24 months—demonstrating maintenance of therapeutic effect achieved with Romo—and are consistent with greater fracture risk reduction observed in this trial with Romo-ALN vs ALN.
To cite this abstract in AMA style:
Brown J, Chines A, Chapurlat R, Foldes J, Nogues X, Civitelli R, De Villiers T, Massari F, Zerbini C, Yang W, Recknor C, Libanati C. Romosozumab Improves Lumbar Spine Bone Mineral Density and Bone Strength Greater Than Alendronate as Assessed by Quantitative Computed Tomography and Finite Element Analysis in the ARCH Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/romosozumab-improves-lumbar-spine-bone-mineral-density-and-bone-strength-greater-than-alendronate-as-assessed-by-quantitative-computed-tomography-and-finite-element-analysis-in-the-arch-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/romosozumab-improves-lumbar-spine-bone-mineral-density-and-bone-strength-greater-than-alendronate-as-assessed-by-quantitative-computed-tomography-and-finite-element-analysis-in-the-arch-trial/