Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science (2591–2596)
Session Type: Abstract Session
Session Time: 1:15PM-1:30PM
Background/Purpose: Transition from long-term denosumab to PTH-analogs or romosozumab might expose patients to the risk of the so-called rebound phenomenon. Adding romosozumab to denosumab might represent an option in patients experiencing a fracture while on denosumab. The aim of this study was to investigate the effects of the combination of romosozumab to denosumab in post-menopausal osteoporosis.
Methods: We did a 36-month prospective, quasi-experimental, study. Post-menopausal women were divided into two groups: patients on denosumab who added romosozumab to denosumab (Dmab from M-24 to M0 à Dmab+Romo from M0 to M+12), and matched controls on denosumab continuing denosumab (Dmab from M-24 to M0 à Dmab from M0 to M+12). Patients for this group were selected from the overall pool of post-menopausal women treated with denosumab referring to our outpatient clinic who had available baseline (M-24) and follow-up DXA scans and serum samples collected (n=388). Patients were then matched through propensity score matching to balance baseline characteristics between the treated and control groups. Bone mineral density (BMD) and bone turnover markers (CTX, P1NP) were assessed at follow-up time points.
Results: A total of 50 women were included in the study. Twenty-five patients in the Dmab à Dmab+Romo group and 25 matched controls in the Dmab à Dmab group. Overall, lumbar spine BMD increased more in the Dmab à Dmab+Romo group (11.6% SE 3.0, p 0.010) compared to the Dmab à Dmab group (8.3% SE 3.3, p NS). Adding romosozumab to denosumab increased P1NP significantly between M0 and M+3 (+22.5 ng/mL SE 8.7, p 0.028). Figure 1 shows the bone markers trends in the two groups, Figure 2 shows the BMD trends in the two groups.
Conclusion: Ongoing treatment with denosumab did not blunt the anabolic response of romosozumab, indicating sustained modeling-based bone formation activity. Adding romosozumab in patients failing denosumab might be a valuable option.
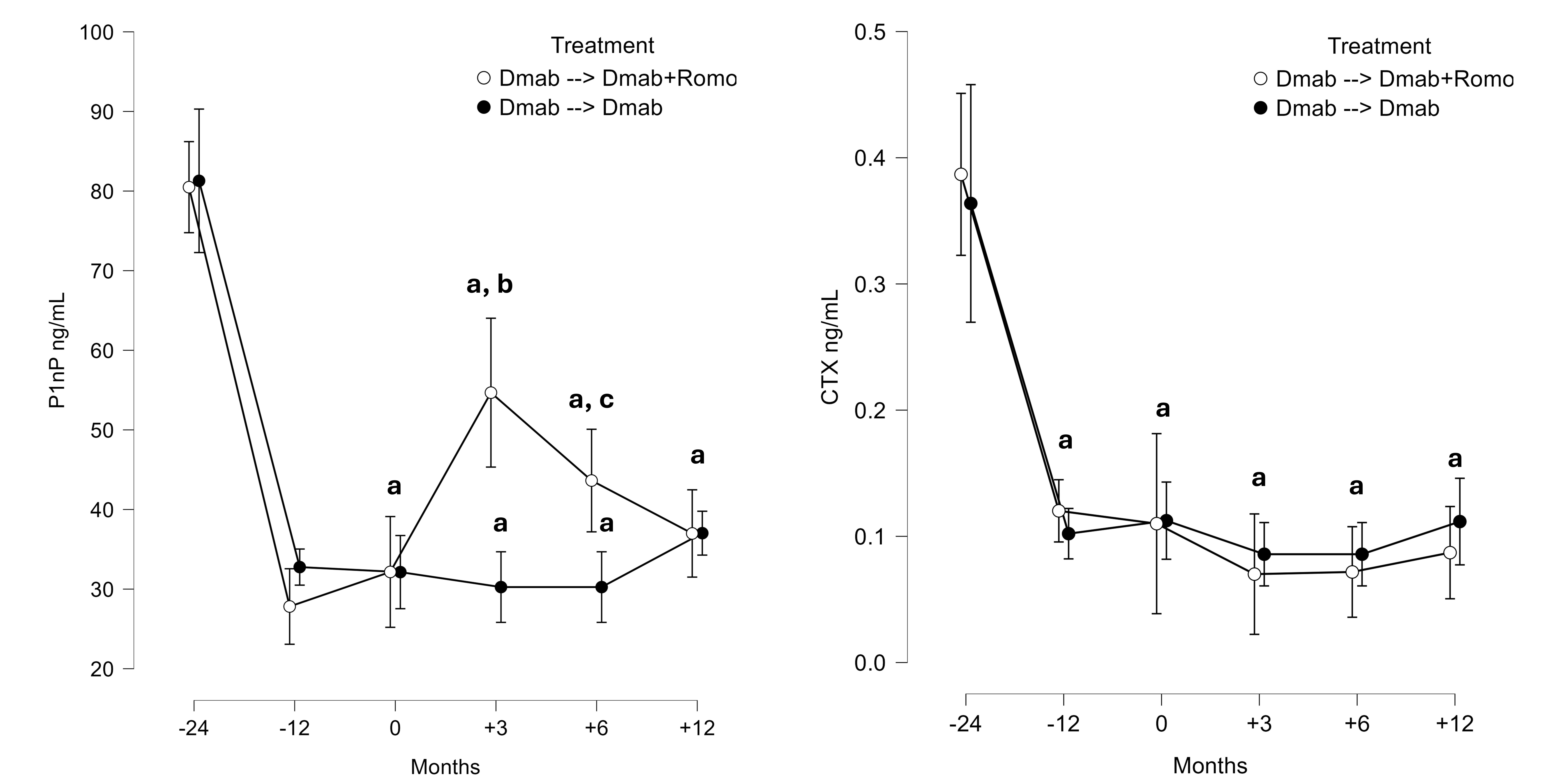 Figure 1. Bone turnover markers (BTMs) changes over the study period
Figure 1. Bone turnover markers (BTMs) changes over the study period
.jpg) Figure 2. Bone mineral density (BMD) changes over the study period
Figure 2. Bone mineral density (BMD) changes over the study period
To cite this abstract in AMA style:
Adami G, Pollastri F, Fassio A, Benini C, Gatti D, Viapiana O, Rossini M. Romosozumab and Denosumab Combination Therapy in Postmenopausal Osteoporosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/romosozumab-and-denosumab-combination-therapy-in-postmenopausal-osteoporosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/romosozumab-and-denosumab-combination-therapy-in-postmenopausal-osteoporosis/
