Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Sclerostin is an osteocyte-derived inhibitor of osteoblast activity. Romosozumab, a monoclonal antibody to sclerostin stimulates bone formation and decreases bone resorption. In a phase 2 study, romosozumab administered for 12 months increased areal bone mineral density (BMD) at the lumbar spine (LS) and total hip (TH) as measured by dual X-ray absorptiometry compared with placebo (Pbo), alendronate, and teriparatide (TPTD) in postmenopausal women with low bone mass. Here we describe the effect of romosozumab on LS and TH volumetric BMD (vBMD), and bone mineral content (BMC) as measured by quantitative computed tomography (QCT) in this trial.
Methods: This international, randomized, Pbo-controlled, phase 2 study enrolled postmenopausal women of 55‒85 years with LS, TH, or femoral neck T‑score ≤–2.0 and ≥–3.5. Measurements with QCT were performed at the “total” LS (mean of L1 and L2 entire vertebral bodies) and TH in subjects receiving Pbo, subcutaneous TPTD (20 μg QD), and romosozumab (210 mg QM). Percentage change from baseline in integral and cortical vBMD and BMC, and trabecular vBMD was evaluated at 12 months. The analyses included subjects with baseline and ≥1 post-baseline QCT measurements.
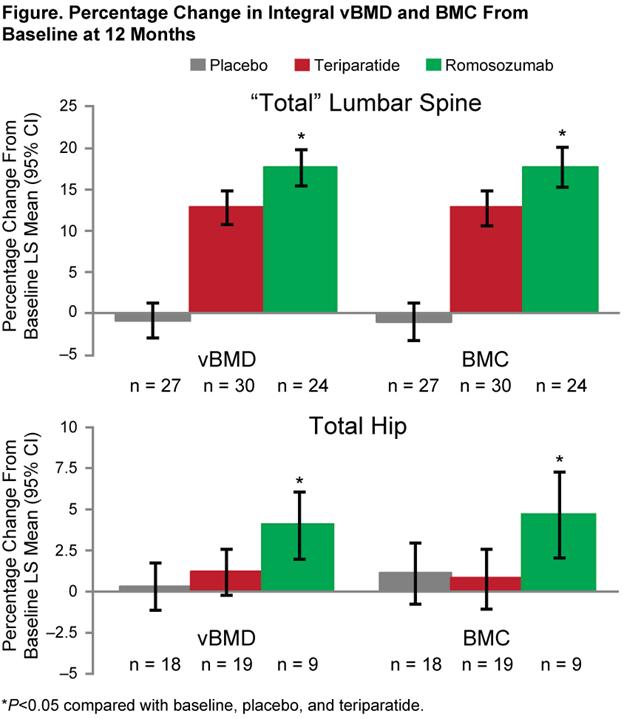
Results: Treatment with romosozumab resulted in significant increases in integral vBMD and BMC at the “total” LS and TH from baseline, and compared with Pbo and TPTD (Figure). TPTD and Pbo were similar at the TH, but not the LS. In the trabecular and cortical bone compartments, differences between romosozumab and TPTD were observed. Trabecular vBMD increased from baseline with both romosozumab and TPTD at the LS and TH (both P<0.05). These gains in trabecular vBMD were similar with romosozumab and TPTD at the LS (18.3% vs 20.1%, respectively), but significantly larger with romosozumab at the TH (10.8% vs 4.2%, P=0.01). Cortical vBMD gains were larger with romosozumab compared with TPTD at the LS (13.7% vs 5.7%, P<0.0001) and TH (1.1% vs –0.9%, P=0.12). Cortical BMC gains were larger with romosozumab compared with TPTD at both the LS (23.3% vs 10.9%, P<0.0001) and TH (3.4% vs 0.0%, P=0.03).
Conclusion: Romosozumab significantly increased vBMD and BMC at the “total” LS and TH compared with Pbo and TPTD in postmenopausal women with low bone mass. The gains, observed in the trabecular and cortical compartments, support the continued clinical investigation of romosozumab as a potential treatment for women with postmenopausal osteoporosis with established BMD deficits and at increased fracture risk.
Disclosure:
H. K. Genant,
Synarc Inc.,
1,
Amgen Inc., Janssen, Lilly, Merck, Novartis, Pfizer, Radius, Servier, and Synarc Inc.,
5,
Merck and Novartis,
9;
S. Boonen,
Amgen Inc. and Novartis,
2;
M. A. Bolognese,
Amgen Inc., Lilly, and Genentech,
2,
Lilly, NOF, and VIVUS,
5;
C. Mautalen,
Merck and Servier,
5;
J. P. Brown,
Amgen Inc., Eli Lilly, Merck, and Novartis,
5,
Abbott, Amgen Inc., Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roche, sanofi-aventis, Servier, Takeda, and Warner Chilcott,
2;
C. Recknor,
Ion Med Systems,
1,
Novartis,
5;
S. Goemaere,
None;
K. Engelke,
Synarc Inc.,
1,
Synarc Inc.,
3,
German government,
2,
Amgen Inc.,
5;
Y. C. Yang,
Amgen Inc.,
1,
Amgen Inc.,
3;
M. Austin,
Amgen Inc.,
1,
Amgen Inc.,
3;
A. Grauer,
Amgen Inc.,
1,
Amgen Inc.,
3;
C. Libanati,
Amgen Inc.,
1,
Amgen Inc.,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/romosozumab-administration-is-associated-with-significant-improvements-in-lumbar-spine-and-hip-volumetric-bone-mineral-density-and-content-compared-with-teriparatide/