Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Available immunosuppressive treatments for autoimmune inflammatory rheumatic diseases (AIRD) might increase the risk for opportunistic infections, such as Pneumocystis jirovecii pneumonia (PJP). Prophylactic trimethoprim-sulfamethoxazole (TMP/SMX) is often prescribed to prevent the occurrence of PJP among patients with AIRD. We calculate the incidence of PJP, PJP-related mortality, and TMP/SMX-related adverse events among patients with AIRD who received a prednisolone dose equivalent of ≥20 mg/day.
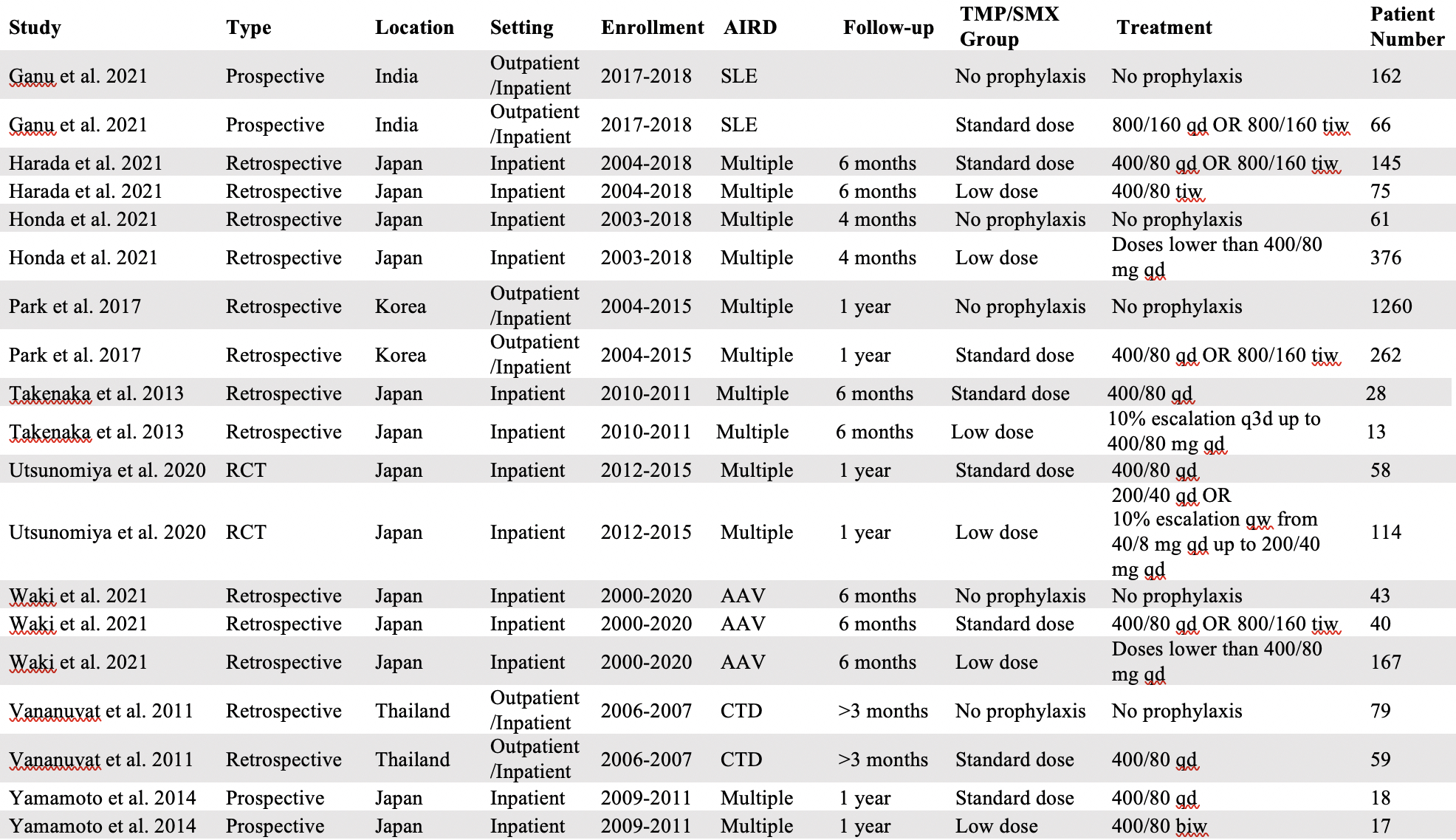
Methods: We searched PubMed and EMBASE for multiple-arm studies evaluating the efficacy and safety of TMP/SMX prophylaxis in individuals with AIRD. We stratified different prophylactic dose regimens of TMP/SMX in two groups: a “low-dose” group if the weekly dosage was lower than the equivalent of 400/80 mg/day, and a “standard-dose” group if the weekly dosage was equal or higher than the equivalent of 400/80 mg/day.
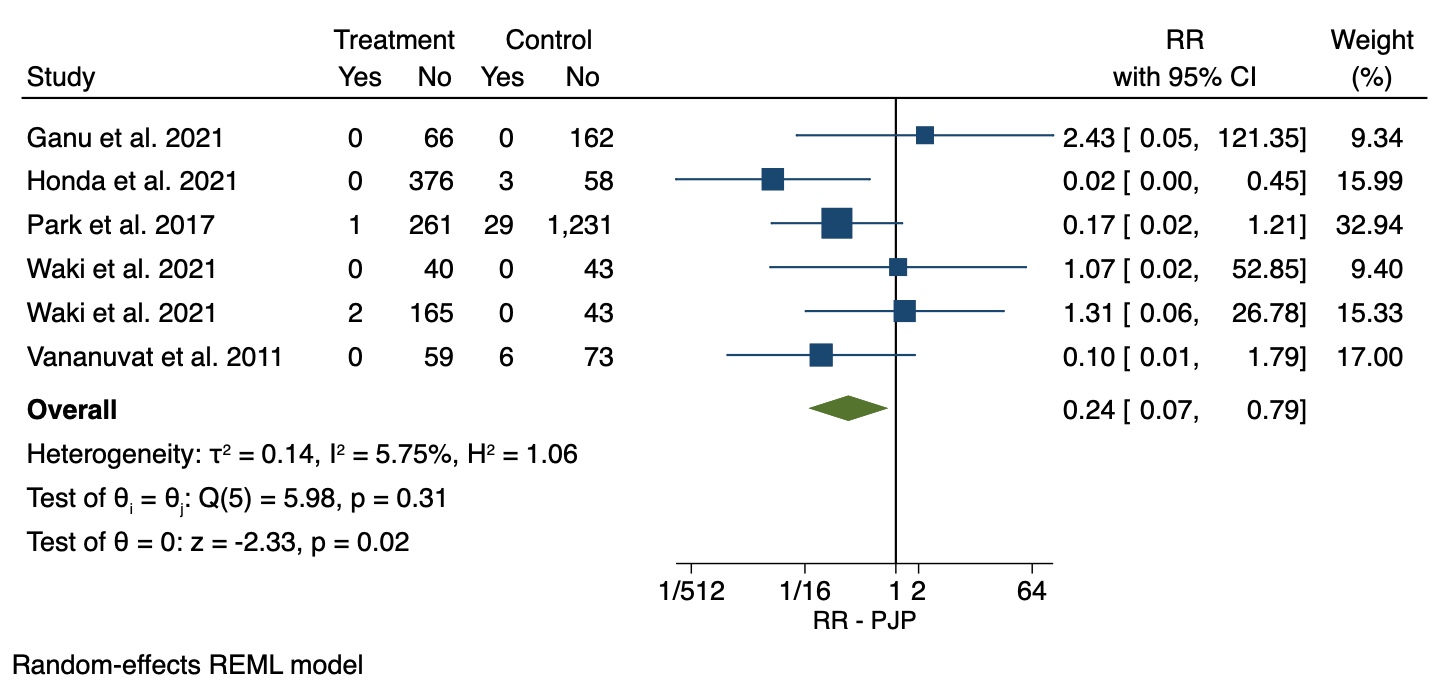
Results: Nine studies, performed in Japan, Korea, Thailand, and India, provided data on 1,438 individuals with AIRD receiving TMP/SMX and 1,605 individuals with AIRD not receiving prophylaxis. Studies were published between 2013 and 2021 and participants were followed from 3 to 12 months (77.75% for more than 6 months). At least one TMP/SMX treatment arm (i.e., “standard-dose”, “low-dose” or both) was present in any included study. Specifically, eight studies included participants grouped in our “standard-dose” group, 6 studies included participants grouped in our “low-dose” group and 5 studies included participants that received no prophylaxis. The relative risk of PJP was 76% lower (Relative Risk 0.24; 95% CI: 0.07-0.79) among participants who received prophylactic TMP/SMX at any dose, compared to patients who did not receive any prophylaxis. The incidence of PJP for the initial 3-12 months of high-dose glucocorticoid treatment among patients who received no prophylaxis (2.95%, 95% CI: 0.65-6.46%) was significantly higher compared to the incidence of PJP among patients who received “standard-dose” (0.00%, 95% CI: 0.00-0.37%, p-value=0.009) or “low-dose” (0.00%, 95% CI: 0.00-0.04%, p-value=0.003) TMP/SMX. PJP-related mortality was 0.62% (95% CI: 0.00-1.98%) among individuals who received no prophylaxis and 0.00% among patients who received “standard-dose” (95% CI: 0.00-0.06%, p-value=0.073) or “low-dose” TMP/SMX (95% CI: 0.00-0.00%, p-value=0.038). We found the incidence of any TMP/SMX-related adverse event was 23.59% (95% CI: 9.77-40.88%) among patients who received “standard-dose” TMP/SMX and 24.06% (95% CI: 11.90-38.63%) among patients who received “low-dose” TMP/SMX, a non-significant difference (p-value=0.946).
Conclusion: Among individuals with AIRD who were included in studies evaluating the use of prophylactic TMP/SMX, the risk for PJP was lower among participants who received TMP/SMX compared to participants who did not receive prophylaxis. The rate of TMP/SMX-related adverse events did not differ among the low-dose and standard-dose TMP/SMX subgroups. Clinical trials evaluating the efficacy and safety of different prophylactic TMP/SMX dose regimens with long term follow-up are needed to study TMP/SMX utility for preventing PJP among patients with AIRD.
To cite this abstract in AMA style:
Vassilopoulos A, Vassilopoulos S, Shehadeh F, Kalligeros M, Mylonakis E. Role of Pneumocystis Jirovecii Pneumonia Prophylaxis with Trimethoprim-Sulfamethoxazole Among Patients with Autoimmune Inflammatory Diseases Receiving High-Dose Glucocorticoids: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/role-of-pneumocystis-jirovecii-pneumonia-prophylaxis-with-trimethoprim-sulfamethoxazole-among-patients-with-autoimmune-inflammatory-diseases-receiving-high-dose-glucocorticoids-a-systematic-review-an/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/role-of-pneumocystis-jirovecii-pneumonia-prophylaxis-with-trimethoprim-sulfamethoxazole-among-patients-with-autoimmune-inflammatory-diseases-receiving-high-dose-glucocorticoids-a-systematic-review-an/