Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Monocytes undergo phenotype changes when exposed to different microenvironments: the classic proinflammatory M1 phenotype, alternative regulatory M2 phenotype and M2-like phenotype, are each regulated by specific transcriptional factors. Here, we investigate whether monocyte phenotypes change with interlukin-1 (IL-1) blockade in systemic onset juvenile idiopathic arthritis (sJIA). We took samples from RAPPORT (RAndomized Placebo Phase study Of Rilonacept in the Treatment of sJIA) to study the phenotype in monocytes by measuring level of transcriptional factors involved in monocyte polarization before and after treatment with Rilonacept, an IL-1 trap.
Methods: Subjects on the Rilonacept arm receive active drug from week 0 for a total of 24 weeks; subjects on Placebo arm receive placebo for 4 weeks then receive active drug Rilonacept for a total of 20 weeks. Blood samples are obtained at week 0, week 2, week 4, week 14 and week 24. RNA extracted from isolated monocytes is analyzed by real time PCR to measure M1 associated genes: Interferon Regulatory factor (IRF) family IRF5, Signal transducers and activators of transcription (STAT) family STAT1; M2 associated genes IRF4, STAT6, Kruppel-Like Factor 4 (KLF4) and peroxisome proliferator-activated receptor-γ (PPAR-γ). All transcript levels are normalized by the levels of 3 housekeeping genes.
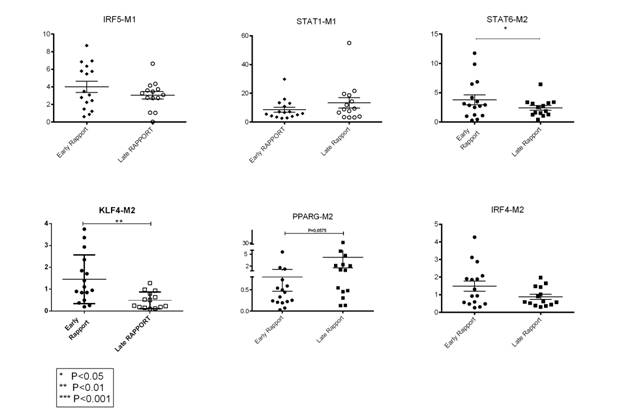
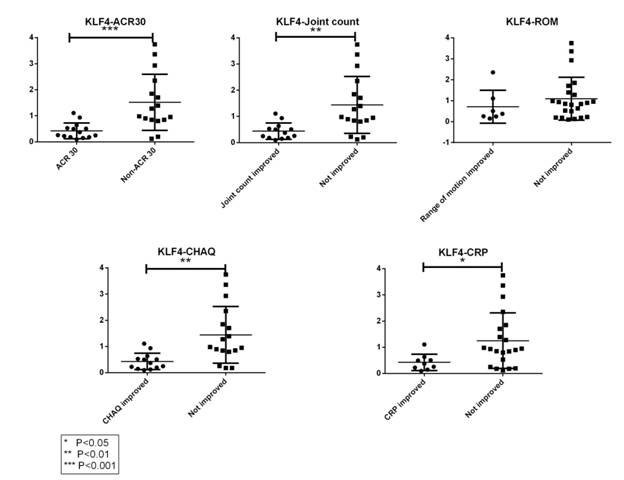
Results: 30 RAPPORT samples from 15 subjects were tested. Samples from subjects treated with Rilonacept for 6 weeks or more (“Late RAPPORT”) showed decreased expression of M2 genes except for PPAR-γ, compared to those treated for less than 6 weeks (“Early RAPPORT”) (see Figure 1). KLF4 showed the most significant decrease in “Late RAPPORT”; in addition, KLF4 is persistently lower in those clinically improved by different measures, including PediACR30 (P=0.0006), joint count (P=0.0024), clinical lab values (P=0.02) or functional state (P=0.0015), but not by improvement in range of motion (ROM) (see Figure 2).
Conclusion: Regulatory M2 genes (KLF4, IRF4 and STAT6) tend to decrease in sJIA monocytes after IL-1 blockade while M2-like gene PPAR-γ appears to increase. There is measurable impact of IL-1 blockade on monocyte phenotype at the transcription factor level.
Figure 1
Figure 2
Disclosure:
Y. Zhang,
None;
C. Macaubas,
None;
C. Klein,
None;
M. V. Pascual,
Novartis Pharmaceutical Corporation,
5,
US Patent,
9,
Novartis Pharmaceutical Corporation,
6;
A. Hay,
None;
S. D. Thompson,
None;
C. I. Sandborg,
None;
N. T. Ilowite,
Novartis Pharmaceutical Corporation,
5,
Novartis Pharmaceutical Corporation,
6,
Janssen Pharmaceutica Product, L.P.,
6;
E. D. Mellins,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/role-of-interleukin-1-in-abnormal-monocyte-phenotype-in-systemic-onset-juvenile-idiopathic-arthritis/