Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease partly characterized by production of autoantibodies, causing inflammation and tissue damage. In this study we investigated whether cumulative genetic risk for elevated immunoglobulin G (IgG)-levels in the general population, represented by an IgG polygenic risk score (IgG-PRS) is associated with an increased risk of SLE, clinical manifestations or autoantibody-profile.
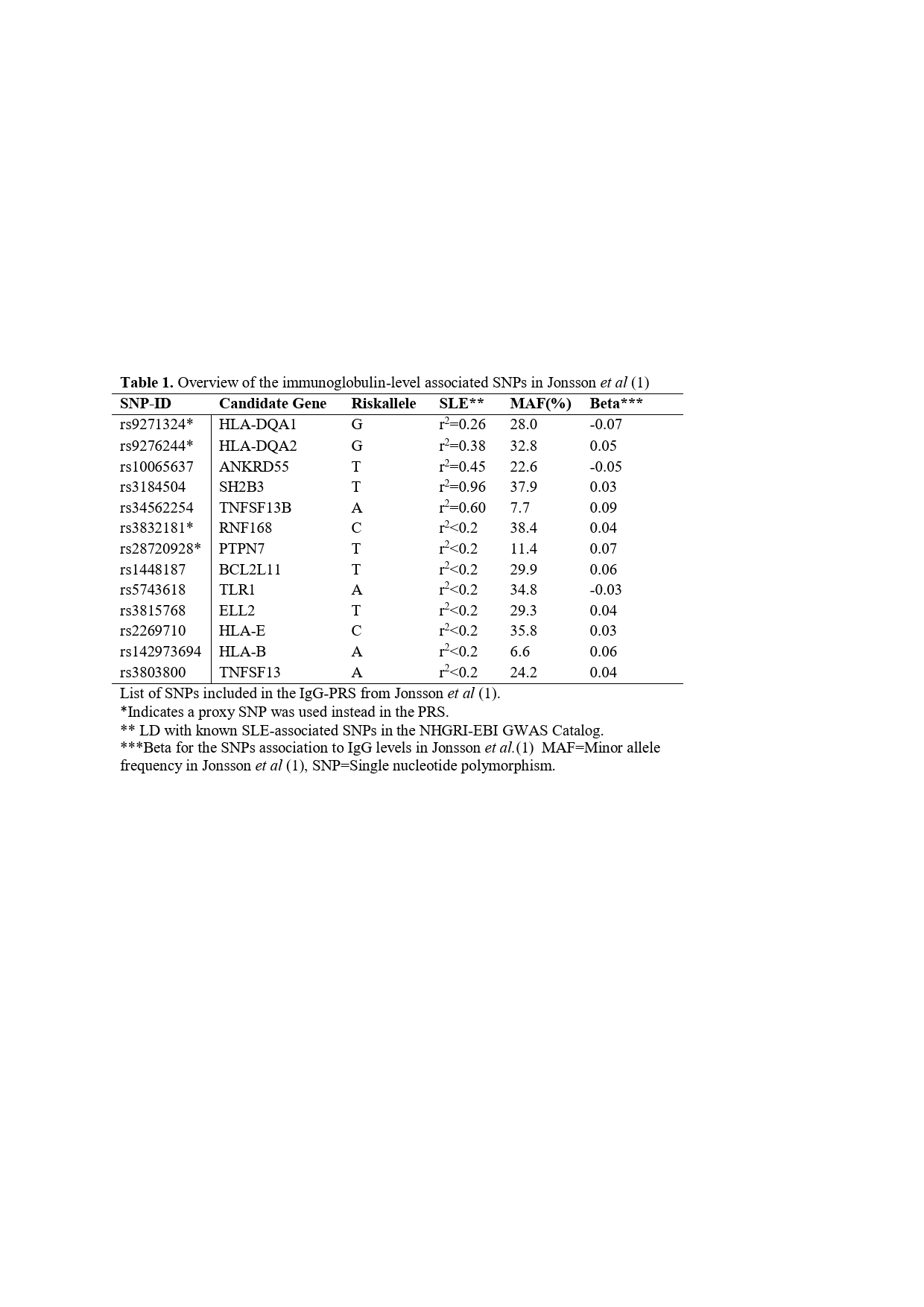
Methods: Patients with SLE, fulfilling the ACR-82, ACR-97 or SLICC-2012 criteria (n=1532) and healthy controls (n=1947) were genotyped using Illumina’s Global Screening Array. Clinical data was retrieved from medical charts. A weighted IgG-PRS was assigned to each individual including 13 single nucleotide polymorphisms (SNPs) associated with elevated IgG-levels in the general population, as reported by Jonsson et al. (1). Next, two sub-PRSs were developed to investigate the SLE and non-SLE genetic effect of the IgG-PRS, one including SLE-associated SNPs (r2 > 0.2, n=5) and another including non-SLE associated SNPs (n=8), table 1. Multiple logistic- and linear regression analyses were performed, adjusting for sex and SLE-duration. P-value < 0.05 was considered significant.
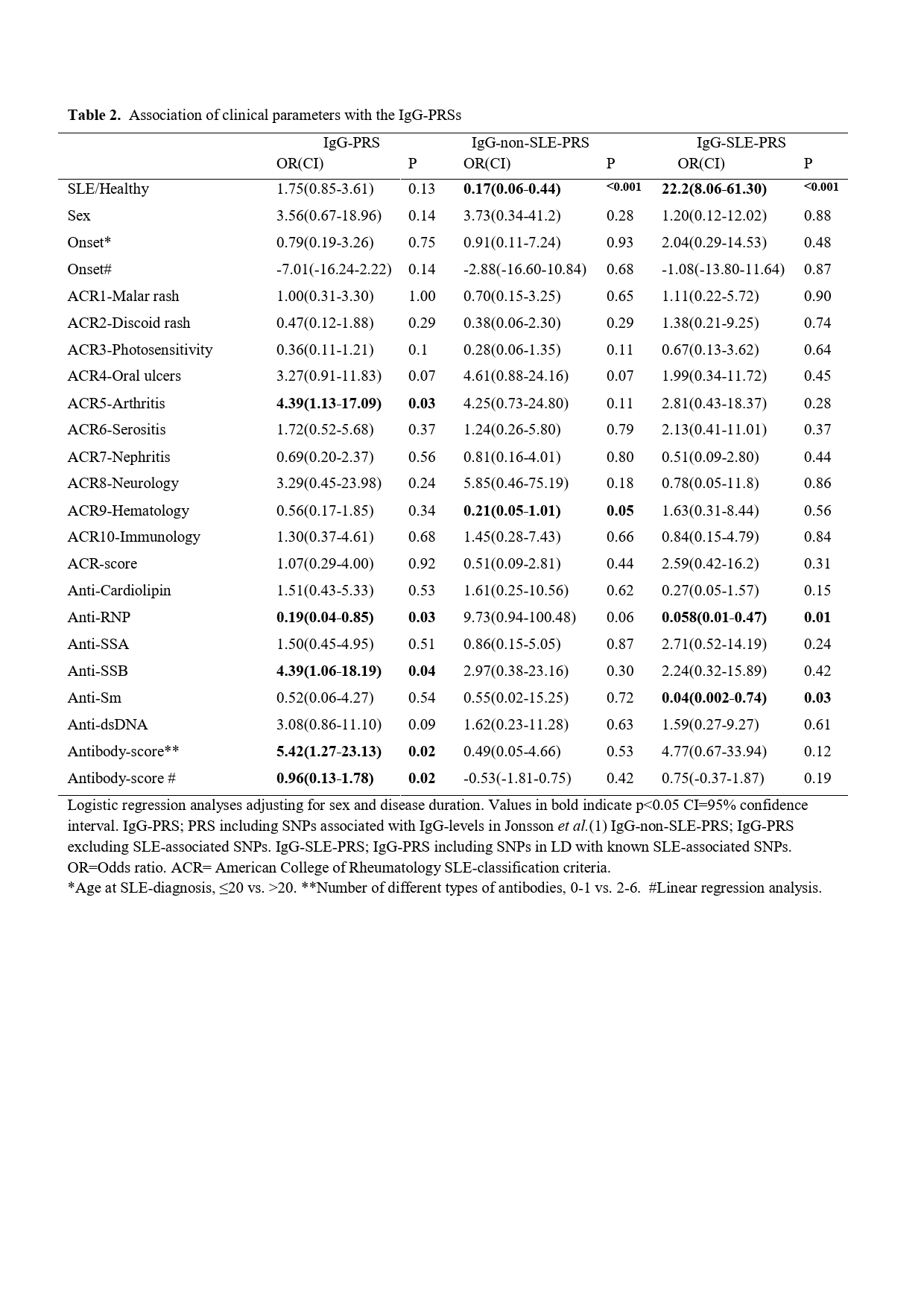
Results: The IgG-PRS did not differ between SLE patients and healthy controls or between men and women (both p >0.05). Out of the ACR-82 criteria, high IgG-PRS was most significantly associated with increased prevalence of arthritis (OR 4.39 (1.13-17.09), p=0.033), table 2. Further, a high IgG-PRS was associated with a greater number of different types of autoantibodies including anti-SSA, anti-SSB, anti-RNP, anti-Sm, anti-dsDNA and anti-cardiolipin antibodies (p=0.02). When investigating the autoantibodies separately, a high IgG-PRS was associated with higher prevalence of anti-SSB (OR 4.39 (1.06-18.19), p=0.04) and lower prevalence of anti-RNP (OR 0.19 (0.043-0.85), p=0.03) antibodies. In total, 38% of the SNPs included in the IgG-PRS were in linkage disequilibrium with SLE-associated SNPs (r2 = 0.26-0.96). Separating the SLE and non-SLE genetic effect for the IgG PRS, demonstrated a positive association for the SLE associated IgG PRS, and conversely a negative association for the IgG PRS excluding SLE associated SNVs (OR 22.2(8.06-61.30), p˂0.001 and OR 0.17(0.06-0.44), p ˂0.001, respectively), table 2.
Conclusion: The cumulative effect of genetic variants affecting IgG-levels in the general population is associated with both the type and the number of different autoantibodies in SLE. Investigating common genetic variants linked to immunological functions in the general population may provide further insight into the genetics driving SLE. References: < !1. Jonsson S, Sveinbjornsson G, de Lapuente Portilla AL et al.Identification of sequence variants influencing immunoglobulin levels. Nature Genetics 49(8):1182-1191, 2017.
To cite this abstract in AMA style:
Gothefors-Holm V, Reid S, Sayadi A, Eloranta M, Frodlund M, Lerang K, Jonsen A, Rantapää-Dahlqvist S, Bengtsson A, Rudin A, Molberg O, Sjöwall C, Sandling J, Rönnblom L, Leonard D. Role of Immunoglobulin Polygenic Risk in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/role-of-immunoglobulin-polygenic-risk-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/role-of-immunoglobulin-polygenic-risk-in-systemic-lupus-erythematosus/