Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: New medicines in SLE have not met endpoints in placebo-controlled RCTs when other evidence indicates they are effective. Reasons suggested are inclusion of patients with inactive disease, inaccurate or subjective outcome measures that seek to address diverse manifestations, comparator arms that include excessive glucocorticoid dose and retention of patients.
Objectives: (i) To evaluate the feasibility of a novel trial design focusing on musculoskeletal SLE with objective eligibility criteria and endpoints and low-dose glucocorticoids (ii) To provide additional validation of LAMDA and ultrasound outcome measures (iii) To provide preliminary evidence for efficacy of rituximab
Methods: Adults with SLE were enrolled if they had clinical synovitis and/or ultrasound (US) tenosynovitis and/or positive power Doppler (PD) in >=1 joint despite stable background therapies including a maximum 10mg prednisolone. Patients were randomized to 1000mg rituximab (Rixathon, RTX) or placebo, on days 1 and 15. Blinded infusions were preceded by 100mg methylprednisolone. Outcome measures, including BILAG-2004, SLEDAI-2K, The Lupus Arthritis and Musculoskeletal Disease Activity Score (LAMDA), tender and swollen joint counts, physician global, patient MSK pain and global VAS, patient reported outcome measures, BICLA, SRI-4 were evaluated monthly. US of both hands and wrists was performed at 0 and 16 weeks. The primary endpoint was overall feasibility. The key efficacy timepoint was 16 weeks. After 16 weeks placebo patients with active disease were eligible for rescue rituximab with repeat follow up timepoints. US and LAMDA were validated against BILAG-MSK improvement at 16 weeks using regression models adjusted for baseline.
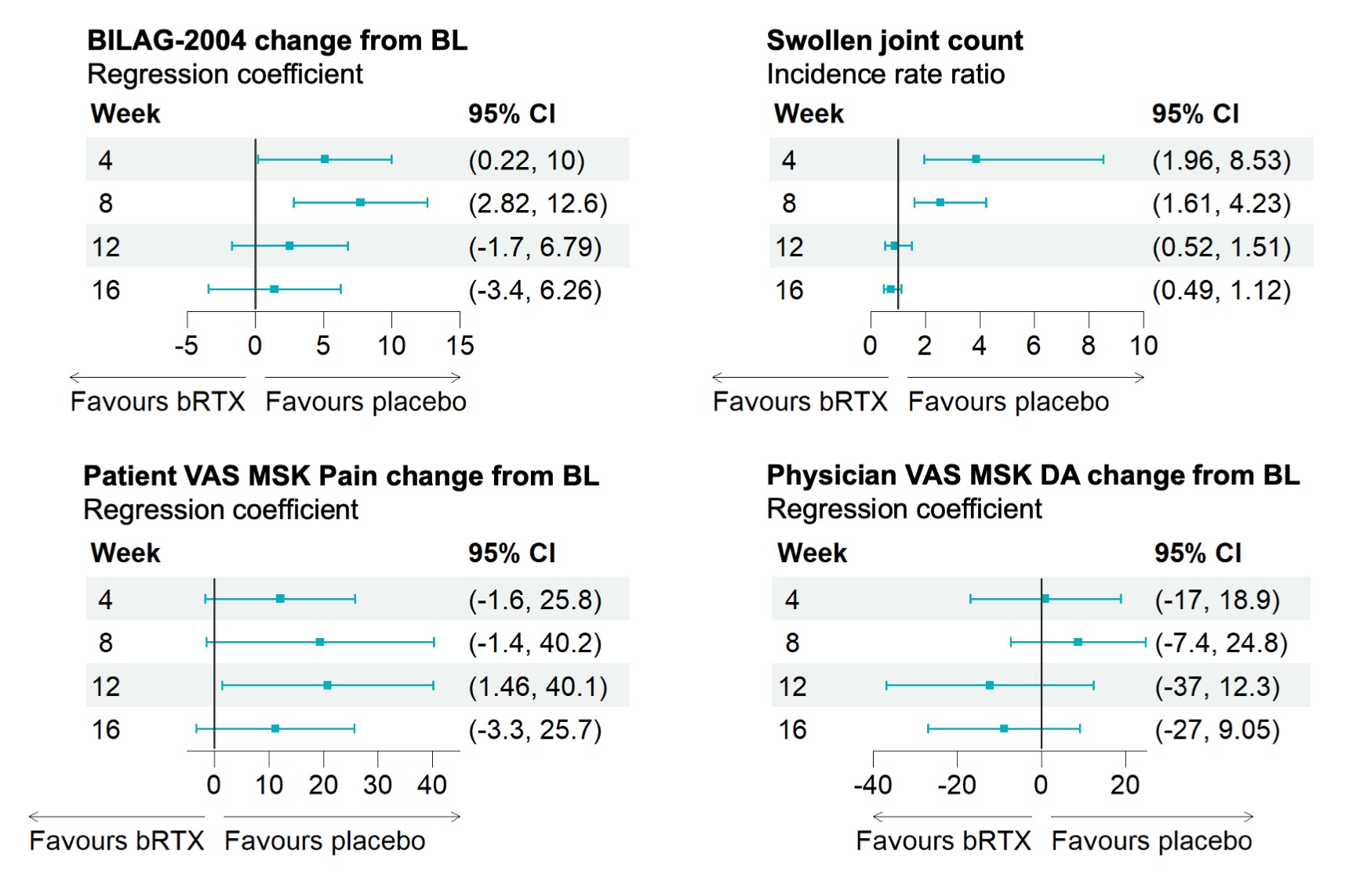
Results: 24/27 (89%) patients were female, 17/27 (63%) were white, 7/27 (26%) were South Asian. Mean (SD) age was 49.7 (12.7) and disease duration 6.7 (9.0). BILAG MSK domain at baseline was scored A in 7/27 (26%), B in 16/27 (59%) and C in 2/27 (7.4%). At 16 weeks, BILAG-MSK response was significantly associated with improvement in LAMDA (OR 0.48, 95% CI 0.18, 0.84); US joints grey-scale (OR 0.56, 95% CI 0.28, 0.85) and US tendons PD (OR 0.33, 95% CI 0.04, 1.01). No substantive difference between arms in efficacy variables was found at week 16. Unexpectedly, results suggested greater improvement in some outcomes in patients who received methylprednisolone and placebo compared to methylprednisolone and rituximab. These measures then converged by 16 weeks (Figure 1) Pooling all rituximab cycles administered (as initial therapy or as rescue) showed improvement in LAMDA (coefficient(95% CI) -2.68 (–4.02, -0.09)), number of joints with US-PD >0 (-0.59(-1.16, -0.02)), and number of joints with GS >1 (-2.68(-4.74, -0.62).
Conclusion: Clinical trials focused on a single feature of SLE (arthritis) are feasible and offer a homogenous trial population, control of standard of care and opportunities for objective imaging. The LAMDA is responsive and associates with validated outcome measures. ROOTS was not powered to measure efficacy but these data suggest potential worsening before benefit from rituximab. Detecting such signals may be enhanced by the greater sensitivity of this trial design.
To cite this abstract in AMA style:
Mahmoud K, Wilson M, Md Yusof M, Brown S, Hensor E, Vital E. Rituximab Objective Outcome Measures Trial in SLE (ROOTS): Randomised and Rescue Therapy Outcomes from a Randomised Controlled Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/rituximab-objective-outcome-measures-trial-in-sle-roots-randomised-and-rescue-therapy-outcomes-from-a-randomised-controlled-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-objective-outcome-measures-trial-in-sle-roots-randomised-and-rescue-therapy-outcomes-from-a-randomised-controlled-trial/