Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Rituximab is one of the many immunosuppressive medications used to treat SLE that can increase a patient’s risk for developing progressive multifocal leukoencephalopathy (PML), a rare but largely fatal neurologic disease. However, despite being one of the few issued with a label warning regarding its association with PML, no study has determined the specific risk rituximab poses among these other medications. We attempted to quantify this risk by retrospectively comparing the prevalence of PML in two matched cohorts of SLE patients with and without previous exposure to rituximab.
Methods: We queried the TriNetX Research Network, a large database of anonymized electronic health records, to identify patients diagnosed with SLE after January 1, 1998. Patients exposed to rituximab or diagnosed with PML prior to this date were excluded. International Classification of Disease (ICD) codes were used to identify diagnoses of SLE and PML, and RxNorm and Healthcare Common Procedure Coding System (HCPCS) codes were used to determine rituximab exposure. Patients with any previous exposure were separated from those without to create the two cohorts. After nearest neighbor propensity score matching, we used Kaplan-Meier curves and the unadjusted hazard ratio (HR) to assess the risk of PML.
Results: The network included 316,307 patients diagnosed with SLE on or after January 1, 1998, without prior orders for rituximab or diagnosis of PML. The prevalence of PML in this initial general population was 0.015%. After propensity score matching, the two cohorts each consisted of 6,452 patients, with fewer than 10 diagnosed with PML in each group. PML occurrence was higher in the rituximab exposed cohort (Figure 1), although the difference was not statistically significant by log-rank test (P = 0.16) or in the proportional hazards model (HR = 2.97, 95% CI 0.60-14.73).
Conclusion: We were unable to demonstrate a statistically significant risk of developing PML from rituximab in patients with SLE when accounting for other comorbidities and immunosuppressive medications. Even assuming the high end of the confidence interval, the number needed to harm patients given rituximab to lead to one case of PML would be high at 513 patients.
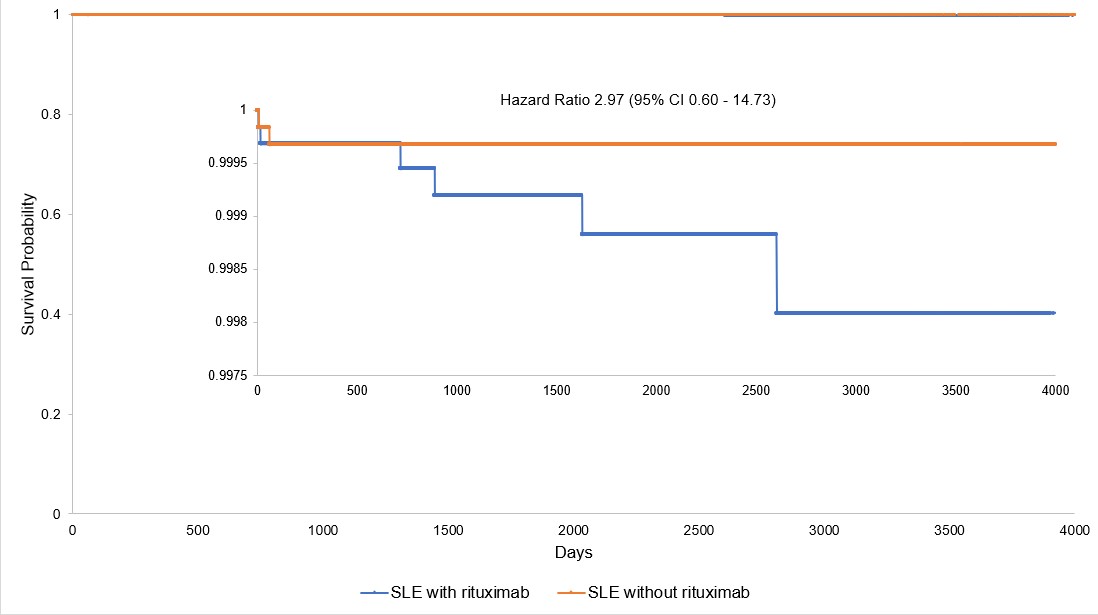 Figure 1. Kaplan-Meier curves and calculated hazard ratio of the two cohorts. The section where the curves diverge has been enlarged and overlayed onto the unaltered graph.
Figure 1. Kaplan-Meier curves and calculated hazard ratio of the two cohorts. The section where the curves diverge has been enlarged and overlayed onto the unaltered graph.
To cite this abstract in AMA style:
Baay B, Nassi L, Most Z. Rituximab Associated Risk for Progressive Multifocal Leukoencephalopathy Among Patients with Systemic Lupus Erythematosus: A Retrospective Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/rituximab-associated-risk-for-progressive-multifocal-leukoencephalopathy-among-patients-with-systemic-lupus-erythematosus-a-retrospective-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-associated-risk-for-progressive-multifocal-leukoencephalopathy-among-patients-with-systemic-lupus-erythematosus-a-retrospective-cohort-study/
