Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Rituximab (RTX) has been commonly used for the treatment of patients with severe or refractory systemic lupus erythematosus (SLE), yet real-world data concerning RTX as the first-line treatment in newly diagnosed moderate-to-severe SLE patients is lacking.
Methods: We conducted a retrospective cohort study using a newly diagnosed (< 3 months) hospitalized Systemic Lupus Inception Cohort (hSLIC) at our center between 1 April 2013 and 1 September 2022. All patients were followed up for at least 12 months or until death. The cohort included patients on RTX (n=104) as the first-line treatment and those on conventional immunosuppressants (IS) (n=154) as comparators. Propensity score-based inverse probability of treatment weighting (IPTW) was used to minimize possible confounding factors. Primary outcome analyses included attainment of modified lupus low disease activity state (mLLDAS) and remission, by 12 months. Secondary outcomes focused on mortality, major flare rates, and the incidence of adverse events of interest, i.e., major infections.
Results: After IPTW, 76.0%/50.5% of RTX-treated patients achieved mLLDAS/remission versus 45.8%/9.7% in the conventional IS group during 12 months follow-up, respectively (p=0.005 and p< 0.001). Sensitivity analyses with renal or neuropsychiatric lupus removal and timeline breakout (pre versus post November 2019) confirmed the robustness of RTX’s efficacy in achieving mLLDAS and remission outcomes. Additionally, the incidence of major infections was similar between the two groups (12.5% vs. 8.4%, p=0.288).
Conclusion: In patients with newly diagnosed moderate-to-severe SLE, upfront treatment with RTX was associated with improved clinical outcomes compared to conventional immunosuppressive therapy, in terms of achieving low disease activity or remission by 12 months.
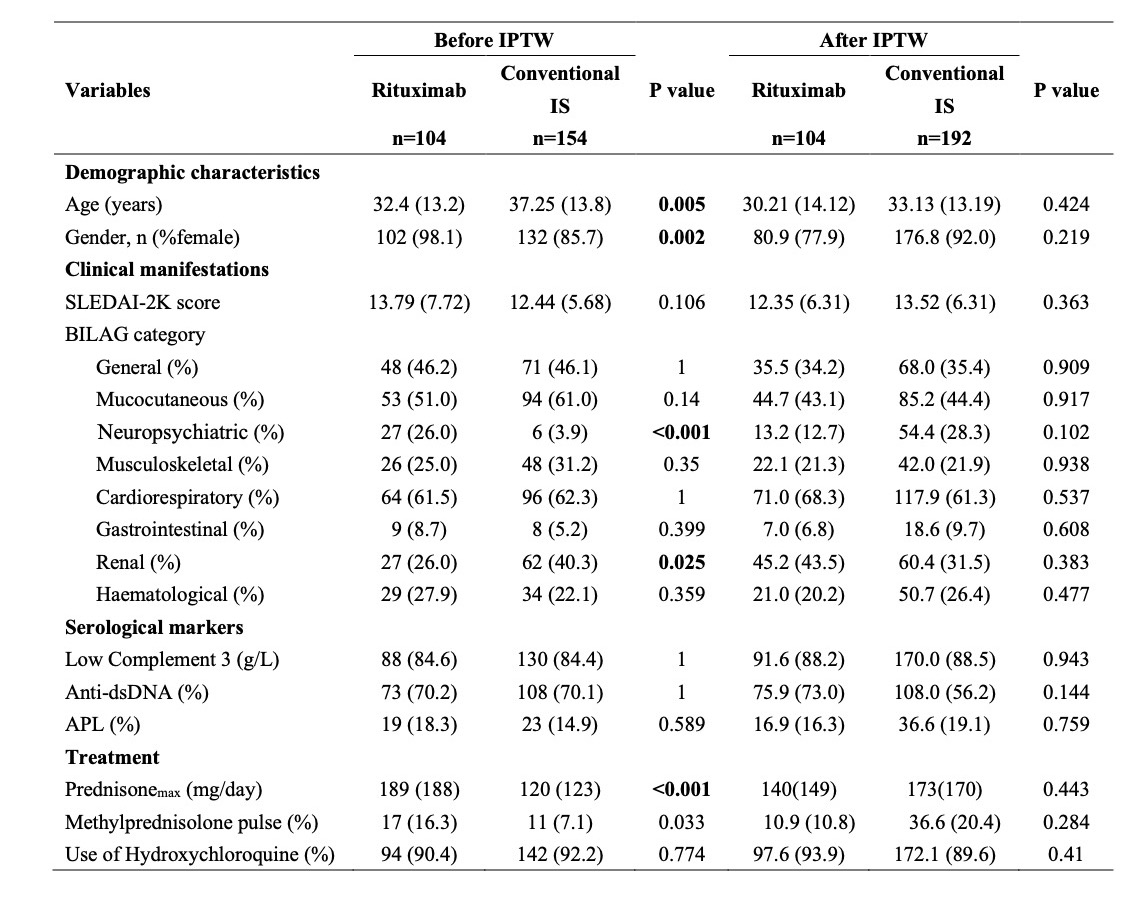 Figure 1. Flow chart of the study.
Figure 1. Flow chart of the study.
Data are number (%) of patients. RTX: rituximab; IS: immunosuppressants; MMF: mycophenolate mofetil; MTX: methotrexate; CTX: cyclophosphamide; AZA: azathioprine; CsA: cyclosporine A; LEF: leflunomide; BLM: belimumab; Pred: prednisone; HCQ: hydroxychloroquine; T2T: treat-to-target; mLLDAS: modified lupus low disease activity state. *Other IS including tripterygium glycoside, tacrolimus, thalidomide and tofacitinib.
.jpg) Table 1. Patient characteristics in the rituximab and conventional immunosuppressant groups before and after IPTW. Data are mean (SD) or number (%) of patients. IPTW: inverse probability of treatment weighting; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; BILAG: British Isles Lupus Assessment Group; IS: immunosuppressants; APL: antiphospholipid antibody; Prednisonemax(mg/day): The highest daily dose of intravenous methylprednisolone administered during the initial hospitalization period; Methylprednisolone pulse (%): Proportion of patients who received intravenous methylprednisolone pulse therapy, defined as ≥500 mg/day for 3 to 5 consecutive days, during the initial hospitalization period.
Table 1. Patient characteristics in the rituximab and conventional immunosuppressant groups before and after IPTW. Data are mean (SD) or number (%) of patients. IPTW: inverse probability of treatment weighting; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; BILAG: British Isles Lupus Assessment Group; IS: immunosuppressants; APL: antiphospholipid antibody; Prednisonemax(mg/day): The highest daily dose of intravenous methylprednisolone administered during the initial hospitalization period; Methylprednisolone pulse (%): Proportion of patients who received intravenous methylprednisolone pulse therapy, defined as ≥500 mg/day for 3 to 5 consecutive days, during the initial hospitalization period.
.jpg) Figure 2. Treat-to-target outcomes. Attainment of mLLDAS (A) and remission (B) within 12 months in the whole cohort and sensitivity analyses (rituximab versus conventional immunosuppressants group). The model was adjusted for age, gender and maximum prednisone dosage. Pre-November 2019 (no COVID-19 exposure): Patients who completed their 12-month follow-up before November 2019. Post-November 2019 (COVID-19 exposure): Patients who completed their 12-month follow-up after November 2019. CI: Confidence interval; mLLDAS: modified lupus low disease activity state; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000. Neuro: Neuropsychiatric; HR: Hazard ratio. At 12-month, follow-up data were missing for 12 out of 258 patients (4.6%) (3 in the RTX group and 9 in the conventional IS group). The last observation carries forward (LOCF) method was employed to address the missing data in the endpoint assessment by 12-month.
Figure 2. Treat-to-target outcomes. Attainment of mLLDAS (A) and remission (B) within 12 months in the whole cohort and sensitivity analyses (rituximab versus conventional immunosuppressants group). The model was adjusted for age, gender and maximum prednisone dosage. Pre-November 2019 (no COVID-19 exposure): Patients who completed their 12-month follow-up before November 2019. Post-November 2019 (COVID-19 exposure): Patients who completed their 12-month follow-up after November 2019. CI: Confidence interval; mLLDAS: modified lupus low disease activity state; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000. Neuro: Neuropsychiatric; HR: Hazard ratio. At 12-month, follow-up data were missing for 12 out of 258 patients (4.6%) (3 in the RTX group and 9 in the conventional IS group). The last observation carries forward (LOCF) method was employed to address the missing data in the endpoint assessment by 12-month.
To cite this abstract in AMA style:
Wang H, Zhao L, Ye S, Yang S. Rituximab as the first line treatment in newly diagnosed systemic lupus erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/rituximab-as-the-first-line-treatment-in-newly-diagnosed-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-as-the-first-line-treatment-in-newly-diagnosed-systemic-lupus-erythematosus/
