Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Maintenance therapy after induction of remission has been shown to reduce relapse rate and mortality in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. The three MAINRITSAN trials have imposed rituximab as gold standard of maintenance treatment. Nevertheless, long-term follow-up of these trials are needed to clarify the risk of late relapse, as well as rituximab optimal schedule and duration.
Methods: The MAINRITSAN randomized controlled trials compared fixed 500 mg rituximab infusion at D0, D14, M6, M12, and M18 with other regimens. All patients enrolled in these trials were prospectively followed until December 2020 and their data were pooled to analyze relapses and adverse events. Data from the three trials were adjusted for variables identified as associated with relapse to make the data from these studies comparable.
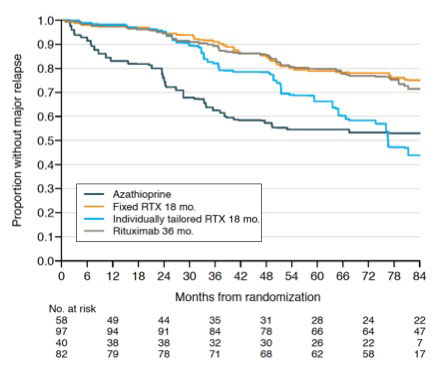
Results: 277 patients were analyzed with a median follow-up of 73 [51-115] months. 203 patients had granulomatosis with polyangiitis (GPA) (73.3%), 196 (70.8%) had newly diagnosed vasculitis, and 255 (92.0%) were ANCA positive, mainly PR3-ANCA (n=170, 61.3%). After adjustment for factors associated with relapse, rituximab remained superior to azathioprine in preventing both overall (major and/or minor) and major relapses (hazard ratio 0.29; 95% CI 0.17-0.50 and 0.40; 95% CI 0.22-0.71, respectively). In contrast, on-demand rituximab administration was associated with an increased risk of major relapse compared with systematic administration (hazard ratio 3.00; 95% CI 1.44-6.27). Finally, prolonging rituximab treatment to 36 months was not associated with a decreased risk of overall or major relapse compared with systematic rituximab administration for 18 months with this extended follow-up update (HR 0.77; 95% CI 0.47-1.26 and 1.16; 95% CI 0.63-2.15, respectively). Long-term follow-up analysis of MAINRITSAN3 taking into account the randomization arm in the MAINRITSAN2 trial confirmed the increased risk of relapse for patients treated with RTX on demand and then placebo compared with patients who received systematic rituximab for 18 months (i.e. patients included in the routine rituximab arm of MAINRITSAN2 and/or rituximab arm of MAINRITSAN3) (major relapse-free survival of patients who received rituximab on-demand followed by placebo 50% (32-78), systematic/placebo 92% (82-100), on-demand/rituximab 93% (80-100), systematic/rituximab 96% (88-100)). 68 patients had serious infections during follow-up (24.5%), and prolonged treatment did not increase serious infections rate (hazard ratio 1.14; 95% CI 0.59-2.23).
Conclusion: The pooled and long-term analysis of patients included in the MAINRITSAN trials confirms that rituximab is effective in preventing relapses in ANCA-associated vasculitis, especially with 500 mg infusions every 6 months for 18 months, without increasing the risk of serious infection. Individually-tailored infusion is associated with an increased risk of major relapse. Finally, extending treatment to 36 months did not appear to be associated with a decreased risk of relapse compared with 18-month regimen.
To cite this abstract in AMA style:
Delestre F, Charles P, Guillevin L, Porcher R, Terrier B. Rituximab as Maintenance Therapy for ANCA-associated Vasculitides: Pooled Analysis and Long-term Outcome of MAINRITSAN Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/rituximab-as-maintenance-therapy-for-anca-associated-vasculitides-pooled-analysis-and-long-term-outcome-of-mainritsan-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-as-maintenance-therapy-for-anca-associated-vasculitides-pooled-analysis-and-long-term-outcome-of-mainritsan-trials/