Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with RA have an increased risk of venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT) compared to non-RA populations1. However, there is limited information available on the risk of VTE among patients receiving treatment with specific DMARDs or categories of therapies. This retrospective cohort study aimed to estimate the incidence of VTE among patients receiving routine clinical care for RA, specifically during treatment with conventional (c) and biologic (b) DMARDs.
Methods: Administrative claims data from Truven Marketscan, with information on patients enrolled in US commercial or Medicare health plans between January 2010 to September 2015, were used to identify patients diagnosed with RA who had a VTE, PE or DVT during an incident treatment episode. Patients were required to maintain enrollment in the health plan and have drug coverage, with a gap of no more than 45 days, and have at least 2 RA diagnosis codes (International Classification of Diseases, 9th Revision, Clinical Modification [ICD 9-CM]) at least 7 days apart, but within a 365-day period. Medication exposures were based on generic medication names and National Drug Codes and included only incident exposures for the specified medications or medication categories. Incident VTE, PE, and DVT were defined using ICD-9-CM codes and, for events diagnosed in outpatient settings, evidence of dispensing of an anticoagulant within 31 days of the event. All analyses were conducted using SAS v.9.4.
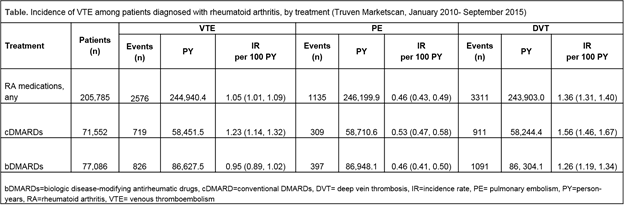
Results: Among 205,785 patients diagnosed with and treated for RA, the crude incidence rate of VTE was 1.05 (95% CI 1.01, 1.09) per 100 person-years (PY). Patients receiving treatment with cDMARDs appeared to be at increased risk of VTE compared to those treated with bDMARDs (see table), but this difference disappeared when age-stratified incidence rates were examined. For PE and DVT, defined based on diagnostic codes only, the crude incidence rates for patients treated with any DMARD were 0.46 (95% CI 0.43, 0.49) and 1.36 (95% CI 1.31, 1.40) per 100 PY, respectively. Incidence rates of VTE were elevated among those with increased age and rates of DVT were consistently higher than rates of PE.
Conclusion: Patients with RA treated with DMARDs were at risk of VTE. Differences in incidence rates of VTE between patients treated with cDMARDs or bDMARDs were likely due to differences in age distributions between groups, with those in the cDMARD cohort tending to be older than those in the bDMARD cohort.
References: 1.Kim SC et al Arthritis Care Res. 2013;65(10):1600
To cite this abstract in AMA style:
Salinas CA, Mitchell L, Giles JT, Dörner T, Motsko SP. Risk of Venous Thromboembolism in Rheumatoid Arthritis Patients in Truven Marketscan Data (Jan 2010–Sept 2015) Treated with Biologic or Conventional Dmards [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/risk-of-venous-thromboembolism-in-rheumatoid-arthritis-patients-in-truven-marketscan-data-jan-2010-sept-2015-treated-with-biologic-or-conventional-dmards/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-venous-thromboembolism-in-rheumatoid-arthritis-patients-in-truven-marketscan-data-jan-2010-sept-2015-treated-with-biologic-or-conventional-dmards/