Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: As many as 30-40% of patients with RA remain on long term glucocorticoids. Infection risk with higher dose glucocorticoids is well known, but evidence on risk with low dose therapy is mixed. We aimed to assess infection risk with low-dose, long-term glucocorticoid use by studying patients on stable DMARD therapy.
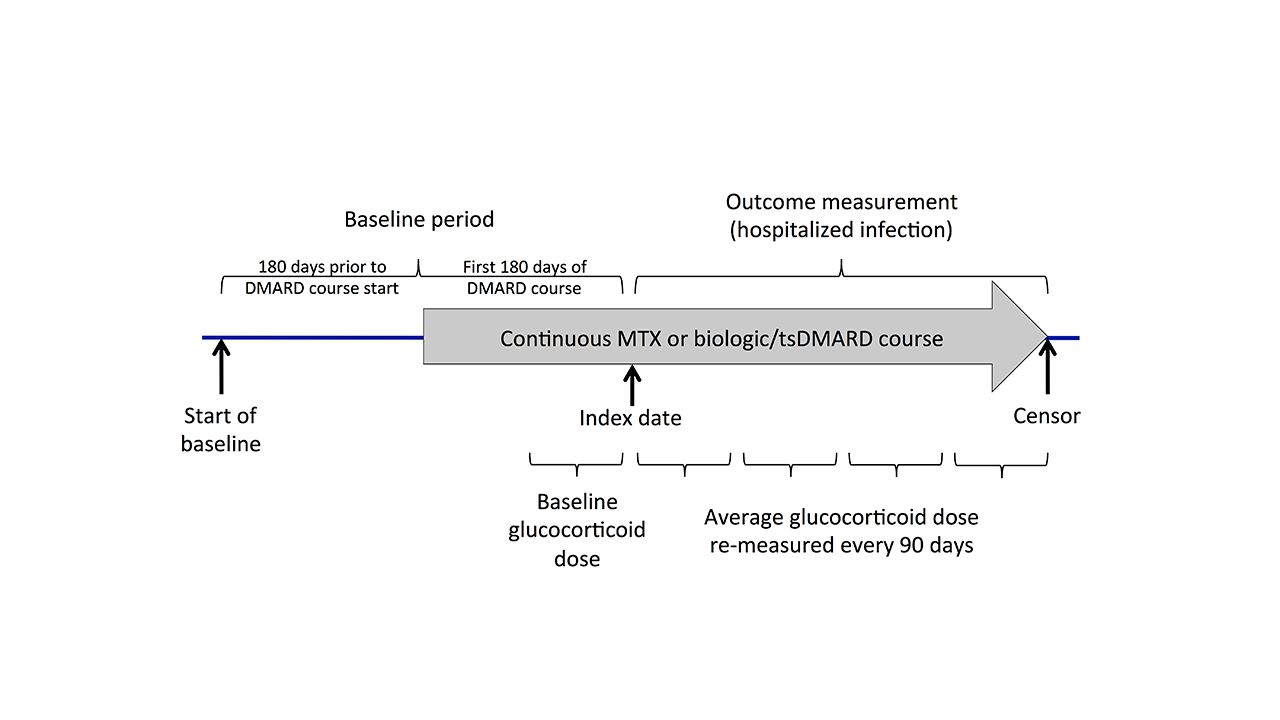
Methods: Using Medicare claims data from 2006-2015, we evaluated RA patients (2 diagnoses ≥7 days apart) who initiated MTX or a biologic/targeted synthetic DMARD (tsDMARD) and remained on therapy for >180 days (no gaps > 90 days and no new biologic/tsDMARDs). Patients with other autoimmune rheumatic diseases, cancer, or HIV were excluded. The baseline period included the 180 days prior to the DMARD course initiation and the first 180 days of treatment (Figure 1). Average glucocorticoid dose in prednisone equivalents was calculated in the final 90 days of the baseline period and updated at each subsequent 90-day interval, categorizing as none, ≤5mg, 5-10mg, or >10mg/day. Cox models evaluated associations between glucocorticoid dose and time to the first serious infection (diagnoses from any position in hospital discharge diagnoses, positive predictive value >80%), clustering to account for patients contributing > 1 treatment episode. Patients were censored at end of the DMARD course, end of enrollment, 9/30/2015, death, or 90 days after a change in glucocorticoid dose category. Covariates were balanced across glucocorticoid dose categories using propensity score-based inverse probability weights. Predicted 1-year incidence of infection was calculated from weighted models.
Results: We identified 244,833 treatment episodes (56% methotrexate and 44% biologic/tsDMARD) among 170,357 unique patients meeting inclusion and exclusion criteria. At baseline 47% of patients were receiving glucocorticoids (Table 1). There were 20,630 serious infections with overall crude incidence of 11.0/100 person-years, most frequently urinary infection, pneumonia, bacteremia/septicemia, and skin/soft tissue infection. Glucocorticoids were associated with an increased rate of serious infections with significant risk even at ≤5mg/day [HR 1.37 (1.32-1.41)] (Table 2). Predicted 1-year incidence of infection from propensity weighted models was 12.5% (95% CI 12.2-12.9) for ≤5mg, 17.2% (16.5-17.9) for 5-10mg, and 23.9% (22.3-25.6) for >10mg vs. 9.3% with no glucocorticoids. Results were similar in sensitivity analyses requiring a primary diagnosis of infection, excluding patients with prior serious infection, and stratifying by MTX vs. biologic/tsDMARD use. Results remained significant in analyses that did not censor with glucocorticoid dose changes (37% of censoring events in the main analysis).
Conclusion: Among older RA patients stable on MTX or biologic/tsDMARDs, long term use of glucocorticoids is associated with a significant increase in the risk of serious infections even at doses ≤5mg/day. The magnitude of risk with ≤5mg/day is similar to that observed with biologics in other studies. Although the influence of disease activity could not be directly assessed, these results support recommendations to limit long term glucocorticoid use in RA.
To cite this abstract in AMA style:
George M, Baker J, Winthrop K, Wu Q, Chen L, Xie F, Yun H, Curtis J. Risk of Serious Infection with Long-Term Use of Low-Dose Glucocorticoids in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-of-serious-infection-with-long-term-use-of-low-dose-glucocorticoids-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-serious-infection-with-long-term-use-of-low-dose-glucocorticoids-in-patients-with-rheumatoid-arthritis/