Session Information
Date: Tuesday, November 7, 2017
Title: Pediatric Rheumatology – Clinical and Therapeutic Aspects Poster III: Juvenile Arthritis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
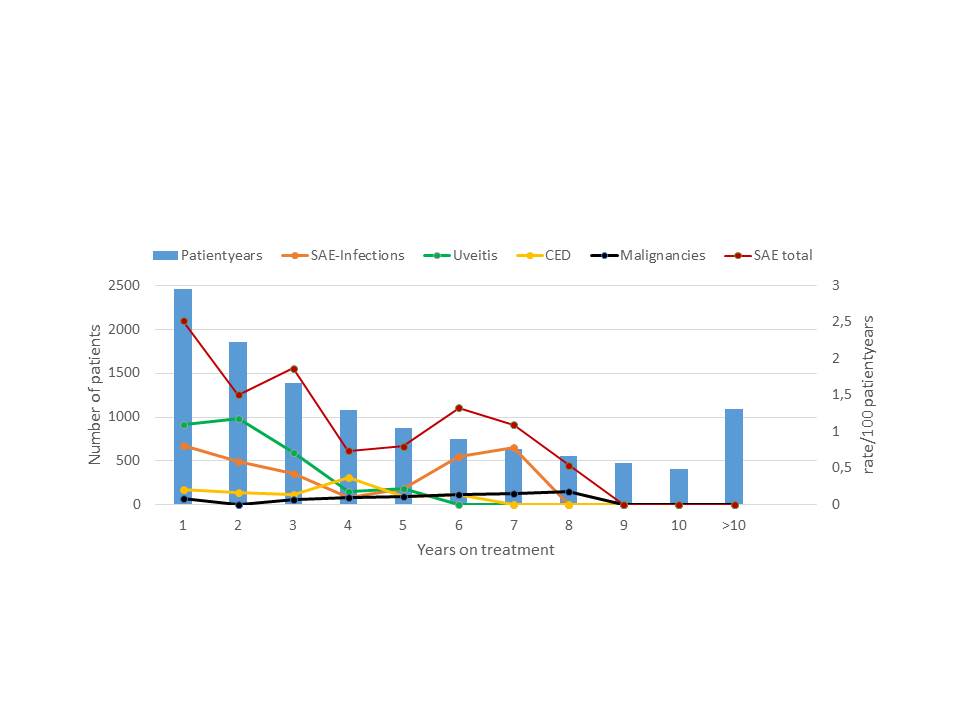
Background/Purpose: To investigate changes in the risk for adverse events of special interest in juvenile idiopathic arthritis (JIA) patients treated with Etanercept documented in the German BIKER registry.
Methods: This prospective cohort study included German JIA patients who began treatment with Etanercept 2000 to 2016 (n = 2,776, 11,772 patient years [PY]). Documentation began at the time of initiation visit and was continued irrespective of the withdrawal of medication. Adverse events (AE) were requested at every visit. AE of special internet included serious infections, uveitis events, diagnosis of chronic inflammatory bowel disease (CED) and malignancies. AE were attributed to Etanercept therapy if they occurred upon treatment or before 90 days of discontinuation. Malignancies were counted as ever exposed.
Results: In total there were 151 serious adverse events (0.054/pt.), 50 serious infections (0.018/pt.), 63 uveitis events (0,023/pat), 16 cases with CED (0.006/pt.) and 8 malignancies (0.003/pt.). The crude incidence rate ratios were 1.3/100 pt-years for all serious events, 0.4/100 pt-years for serious infections, 0.5/100 pt-years for uveitis events, 0.14/100 pt-years for CED and 0.06/100 pt-years for malignancies. The incidence of total serious events in the first year of 2.5/100 pt-years markedly decreased upon continuous treatment as well as the incidence of serious infections (first year 0.81/100PY), uveitis events (first year 1.09/100 PY) while no trend was observed with the rate of CED events (first year 0.20/100 patient years) or the rate for malignancies (0.08/100 patient years). Upon observation for more than 10 years, the yearly incidence markedly decreased for serious infections and uveitis events upon continuous treatment and remained stable for CED and malignancies (figure).
Conclusion : These results indicate significant decrease of the risk for several adverse events of special interest with continuous etanercept treatment over time; this may be explained by an evidence-based risk management for JIA patients given etanercept including the renouncement of steroids in the case of serious infections. Susceptible patients may be recognized early during the course of treatment. No increase of any of the risks was observed upon continuous treatment for up to 16 years.
To cite this abstract in AMA style:
Horneff G, Weller-Heinemann F, Hospach T, Oommen P, Ganser G, Haas JP, Minden K, Klein A. Risk of Serious Events, Serious Infections, Uveitis, Crohn’s Disease, Colitis and Malignancies in Patients with Juvenile Idiopathic Arthritis on Continuous Treatment with Etanercept over Time: A Report from a Biologics Registry [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/risk-of-serious-events-serious-infections-uveitis-crohns-disease-colitis-and-malignancies-in-patients-with-juvenile-idiopathic-arthritis-on-continuous-treatment-with-etanercept-over-time/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-serious-events-serious-infections-uveitis-crohns-disease-colitis-and-malignancies-in-patients-with-juvenile-idiopathic-arthritis-on-continuous-treatment-with-etanercept-over-time/