Session Information
Date: Tuesday, November 9, 2021
Title: RA – Treatments Poster III: RA Treatments & Their Safety (1674–1710)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Initial reports from “ORAL Surveillance” post-marketing safety trial have suggested that tofacitinib, in comparison with tumor necrosis factor inhibitors (TNFI), may be associated with increased risk of malignancies in RA patients. We conducted a multi-database population-based study to further examine this safety concern in real-world settings.
Methods: The ‘Optum’ Clinformatics (2012-2020), IBM ‘MarketScan’ (2012-2018), and Medicare (parts A, B, and D, 2012-2017) claims databases were used to construct two cohorts of RA patients initiating treatment with tofacitinib and TNFI. The first cohort, “real-world evidence” (RWE), included a representative cohort of RA patients treated in routine practice. As a calibration exercise, we mimicked the inclusion and exclusion criteria of the ORAL Surveillance trial which consisted of patients minimum 50 years of age and with at least one cardiovascular risk factor, in the second cohort (“RCT-duplicate”). The primary endpoint, a composite cancer outcome, was defined using previously validated claims-based algorithms for any malignancy excluding non-melanoma skin cancer. In the primary as-treated analysis, patients were followed from treatment initiation until study outcomes, treatment discontinuation or switch, insurance disenrollment, death, or end of the study period, whichever occurred first. Cox proportional hazards models were used to generate hazard ratios (HR) and 95% confidence intervals (CI). Propensity score (PS) fine-stratification weighting accounted for over 60 potential confounders. Database-specific estimates were pooled using fixed effects models with inverse variance.
Results: The RWE cohort included 25,389 patients in Optum, 29,511 in MarketScan, and 28,374 in Medicare of whom 13.0%, 15.3%, and 9.5% initiated treatment on tofacitinib, respectively. The majority of RA patients were female across the three databases (77%-80%). The mean age was 54 and 52 in Optum and MarketScan and 71 in Medicare. The median follow-up time on treatment was 199-218 days. The crude incidence rates (95%CI) per 100 person-years of composite cancer endpoint comparing tofacitinib and TNFI users were 1.65 (1.21-2.19) and 1.36 (1.20-1.52) in Optum, 0.60 (0.39-0.90) and 0.84 (0.73-0.96) in MarketScan, and 2.70 (2.07-3.46) and 2.48 (2.28-2.70) in Medicare. The pooled PS-weighted HR (95%CI) for composite cancer outcome comparing tofacitinib with TNFI was 1.01 (0.83-1.22). The pooled PS-weighted HR (95%CI) was 1.19 (0.86-1.64) in RCT-duplicate cohort (versus Oral Surveillance trial, HR: 1.48, 95%CI: 1.04-2.09). Consistent results were observed in sensitivity analyses implementing a 90- day exposure lag for latency of treatment effect and a180-day grace period for persistence of effect after treatment cessation.
Conclusion: In this large population-based study, tofacitinib, in comparison with TNFI, was not associated with risk of malignancies in RA patients in the real-world setting. However, our results cannot rule out an elevated risk of cancer associated with tofacitinib among patients who were at least 50 years of age with one cardiovascular risk factor. Additional studies with long-term follow-up are required to corroborate these findings.
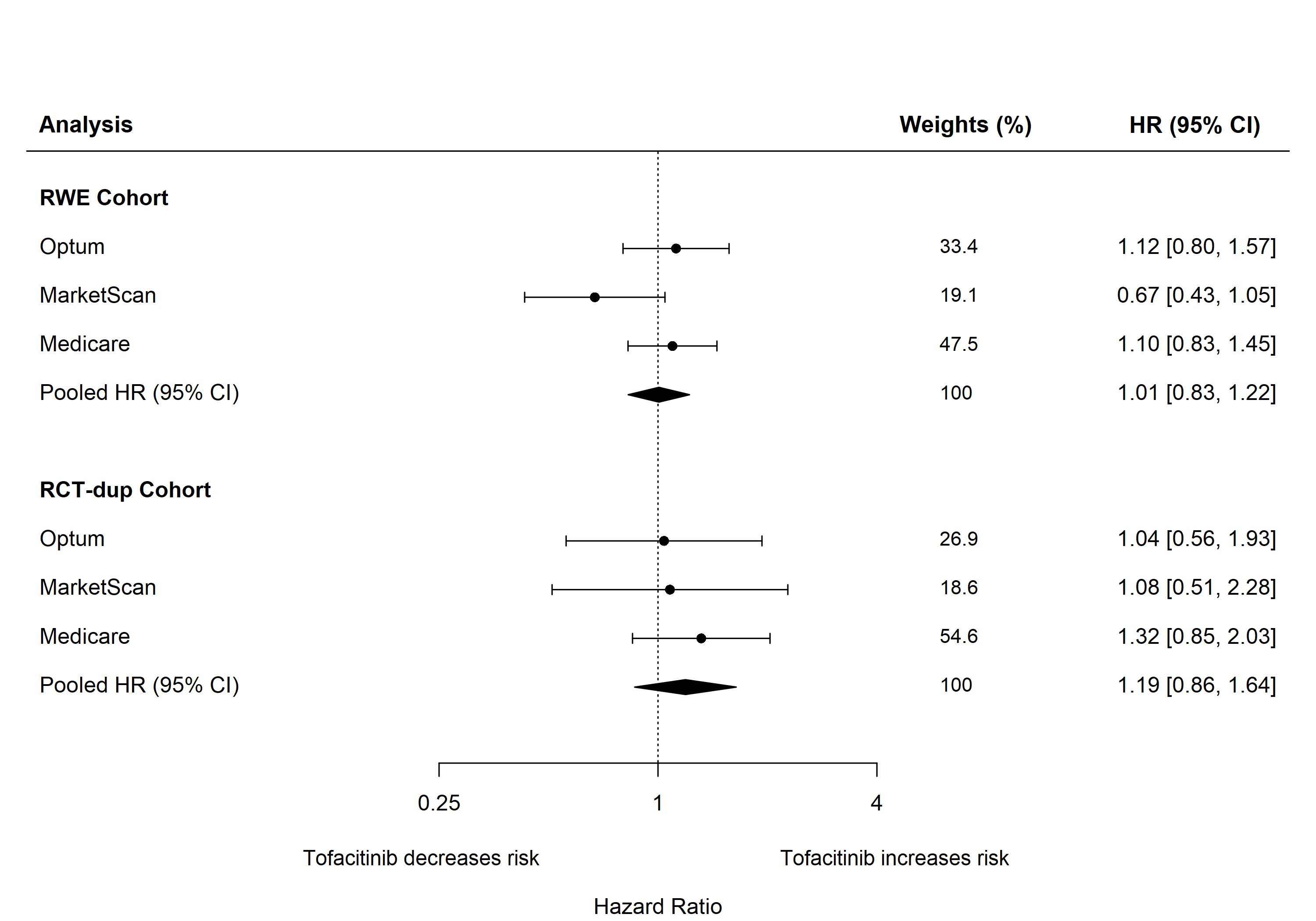 Figure. Risk of composite cancer outcomes when comparing tofacitinib with TNF inhibitors (reference) in patients with RA
Figure. Risk of composite cancer outcomes when comparing tofacitinib with TNF inhibitors (reference) in patients with RA
To cite this abstract in AMA style:
Khosrow-Khavar F, Desai R, Lee H, Lee S, Kim S. Risk of Malignancy in Patients Treated with Tofacitinib: Results from the Safety of TofAcitinib in Routine Care Patients with Rheumatoid Arthritis (STAR-RA) Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/risk-of-malignancy-in-patients-treated-with-tofacitinib-results-from-the-safety-of-tofacitinib-in-routine-care-patients-with-rheumatoid-arthritis-star-ra-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-malignancy-in-patients-treated-with-tofacitinib-results-from-the-safety-of-tofacitinib-in-routine-care-patients-with-rheumatoid-arthritis-star-ra-study/
