Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Inflammatory and demyelinating central nervous system (CNS) adverse events have been observed among new users of tumor necrosis factor (TNF) inhibitors. No studies to date have defined the risk of these adverse events after initiation of TNF inhibitors as compared to other biologic and synthetic disease-modifying antirheumatic drugs (b/tsDMARD).
Methods: Data were derived from the US-based TriNetX electronic health records database. Patients were included if they had a disease for which TNF inhibitors are indicated (rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis) and received a b/tsDMARD with regulatory approval for one of those diseases. Inflammatory CNS events were identified using ICD9-CM/ICD10-CM codes and unadjusted incidences were calculated. The adjusted risk among new users of TNF inhibitors was compared to other b/tsDMARDs using weighted Cox proportional hazards models.
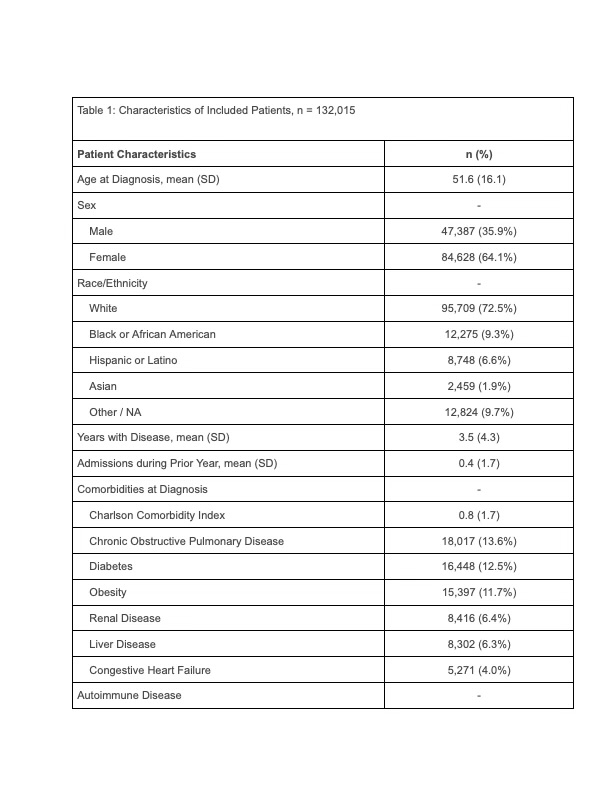
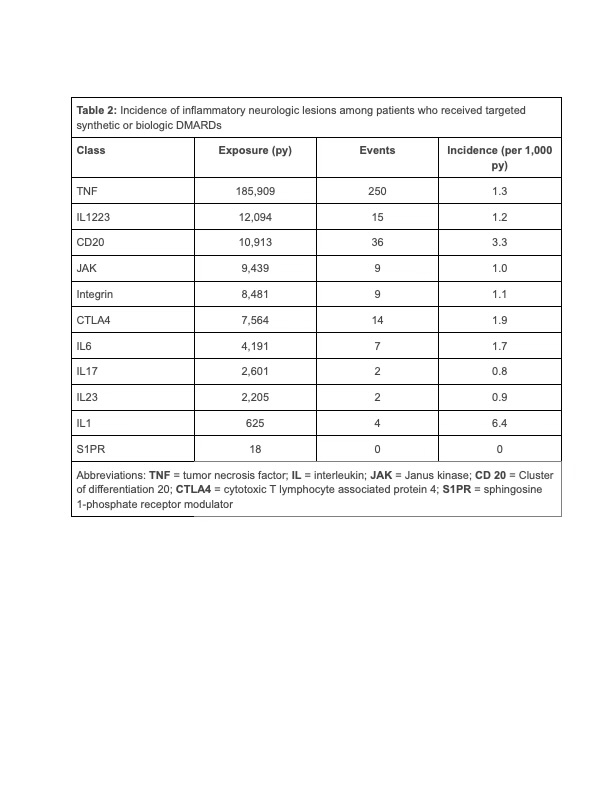
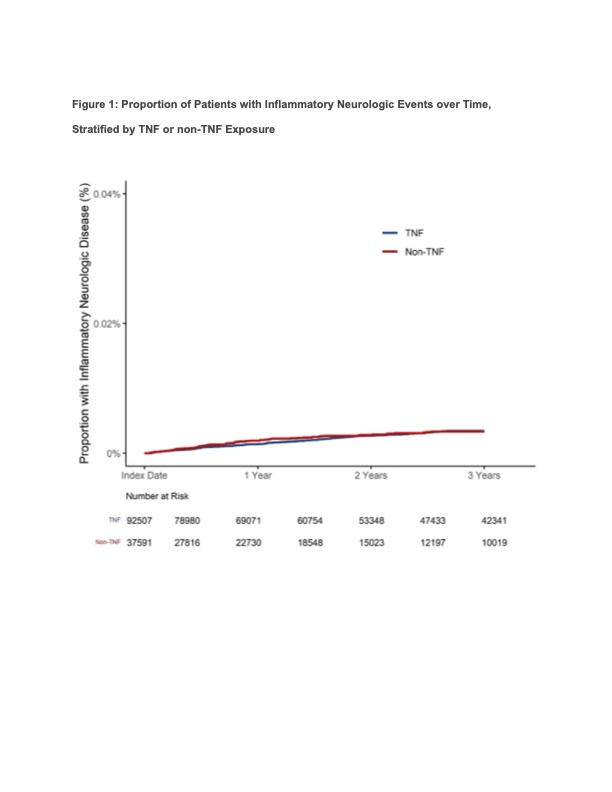
Results: Among 132,015 patients included in the analysis (84,628 female [64.1%]; mean age 51.6 [standard deviation{SD}, 16.1]), the most common first biologic agent was a TNF inhibitor (93,661, [70.9%]) followed by IL 12/23 inhibitor (7,949, [6.0%]) and Janus kinase inhibitors (6,900, [5.2%]). Patients were followed for an average of 1.85 years (SD 1.17 ) and contributed 244,038 patient-years of exposure time. In the primary analysis, there were 348 patients who had an inflammatory CNS event (mean time to event 388 days [SD 285 days]). The unadjusted incidence of inflammatory CNS events was numerically lower among new users of TNF inhibitors (185,909 total exposure years; 250 events, incidence 1.34 per 1,000 patient-years) as compared to the combined non-TNF group (58,130 exposure years, 98 events, incidence 1.69 per 1,000 patient-years).The incidence per 1,000 person-years varied by drug class (Table 2). In a weighted Cox proportional hazards regression with TNF exposure as the referent, there was no association between TNF exposure and inflammatory CNS events as compared to the combined non-TNF b/tsDMARDs (hazard ratio [HR] 1.01, 95% confidence interval [CI] 0.75-1.36). These results persisted in subset analyses of patients with rheumatoid arthritis (HR 1.03, 95% CI 0.67-1.59), psoriasis, psoriatic arthritis, and ankylosing spondylitis (HR 1.02, 95% CI 0.47-2.19), and Crohn’s disease or ulcerative colitis (HR 1.02, 95% CI 0.41-2.57).
Conclusion: This large real-world analysis of patients initiating b/tsDMARDs did not identify an increased risk of inflammatory CNS events among new users of TNF inhibitors compared to other b/tsDMARDs. Meta-analyses of randomized trials should be conducted to corroborate these findings, which may be affected by channeling bias.
To cite this abstract in AMA style:
Casey M, Pannu S, Bajwa S, Duarte-Garcia A, Putman M. Risk of Inflammatory Central Nervous System Diseases Among New Users of Biologic and Targeted Synthetic Disease-Modifying Antirheumatic Drugs [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/risk-of-inflammatory-central-nervous-system-diseases-among-new-users-of-biologic-and-targeted-synthetic-disease-modifying-antirheumatic-drugs/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-inflammatory-central-nervous-system-diseases-among-new-users-of-biologic-and-targeted-synthetic-disease-modifying-antirheumatic-drugs/