Session Information
Date: Sunday, November 12, 2023
Title: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Pre-licensure clinical trials for recombinant zoster vaccine (RZV) identified more frequent gout diagnoses among recipients of RZV than placebo; however, these trials were not powered to assess the statistical significance of these differences. The current real-world safety study used administrative claims data from 4 national insurers and 1 regional insurer in the FDA’s Sentinel System, a large, distributed data network, to assess whether RZV is associated with an increased risk of new-onset gout among US individuals ≥50 years of age
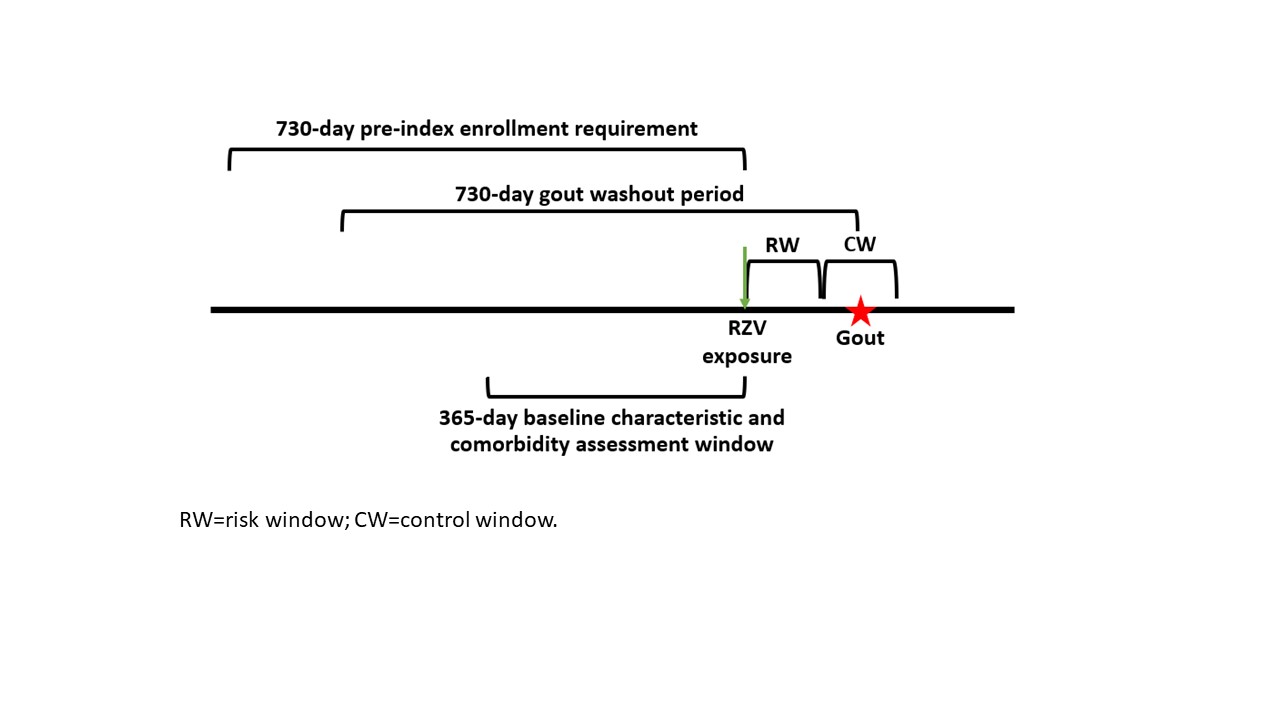
Methods: This retrospective study used a self-controlled risk interval (SCRI) design (Figure 1). We included health plan members ages ≥50 with RZV exposure followed by incident gout within 60 days: days 1-30 defined the risk window (RW) and days 31- 60 defined the control window (CW). We required 730 days of medical and drug coverage, allowing 45-day gaps, prior to RZV exposure, and 60 days of coverage, with no gaps, after RZV exposure. A conditional Poisson model estimated the risk ratio (RR) of gout in the RW versus CW.
The exposure was receipt of at least one of the two-dose RZV regimen between January 2018 through December 2019, identified by Current Procedural Terminology (CPT) or National Drug Code (NDC). Incident gout was defined as a gout diagnosis followed by a dispensing of allopurinol, colchicine, probenecid, or febuxostat within 3 months, with no gout diagnoses or medications in the 730 days prior. Baseline characteristics (e.g., comorbidities) were evaluated in the 365 days prior to the RZV date.
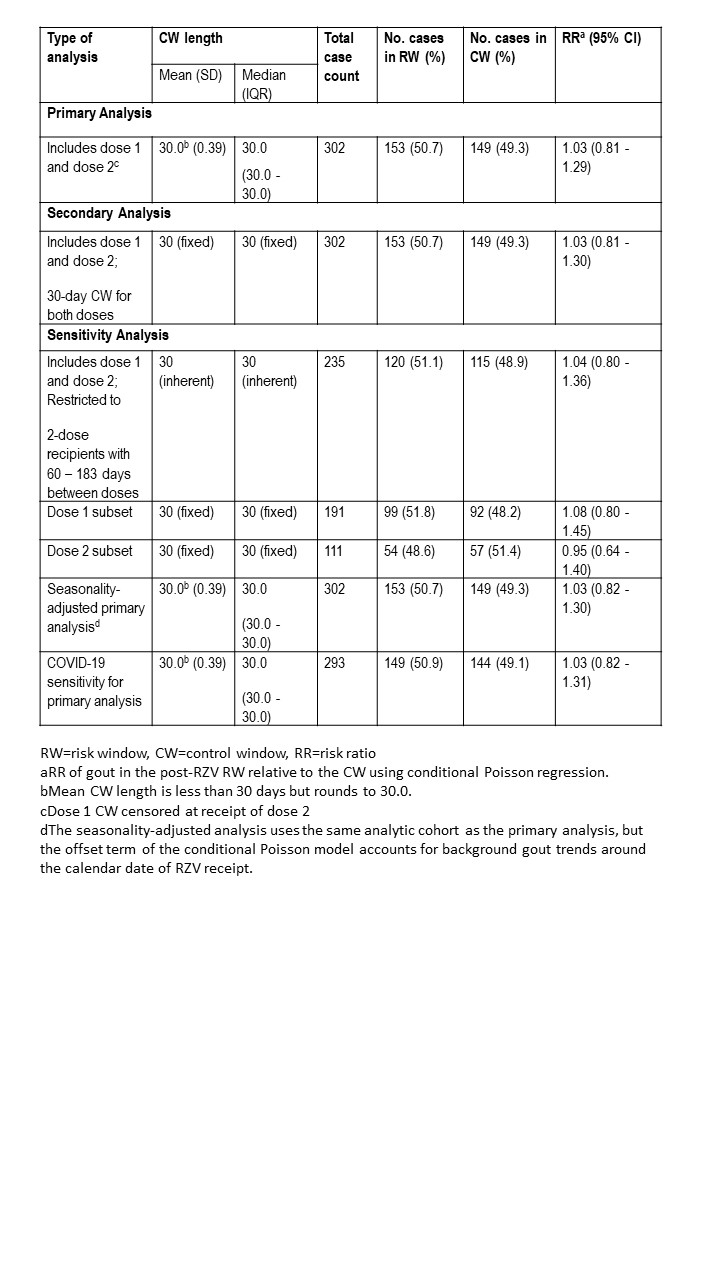
The primary analysis assessed the risk of new-onset gout following any RZV dose, censoring the dose 1 CW at dose 2 receipt. The secondary analysis assumed uniform 30-day CWs. Sensitivity analyses evaluated dose 1- and dose 2-specific risks, risk among patients compliant with recommended dose spacing of 60–183 days, adjustment for seasonality, and restriction to the pre-COVID era (excluding cases after December 1, 2019).
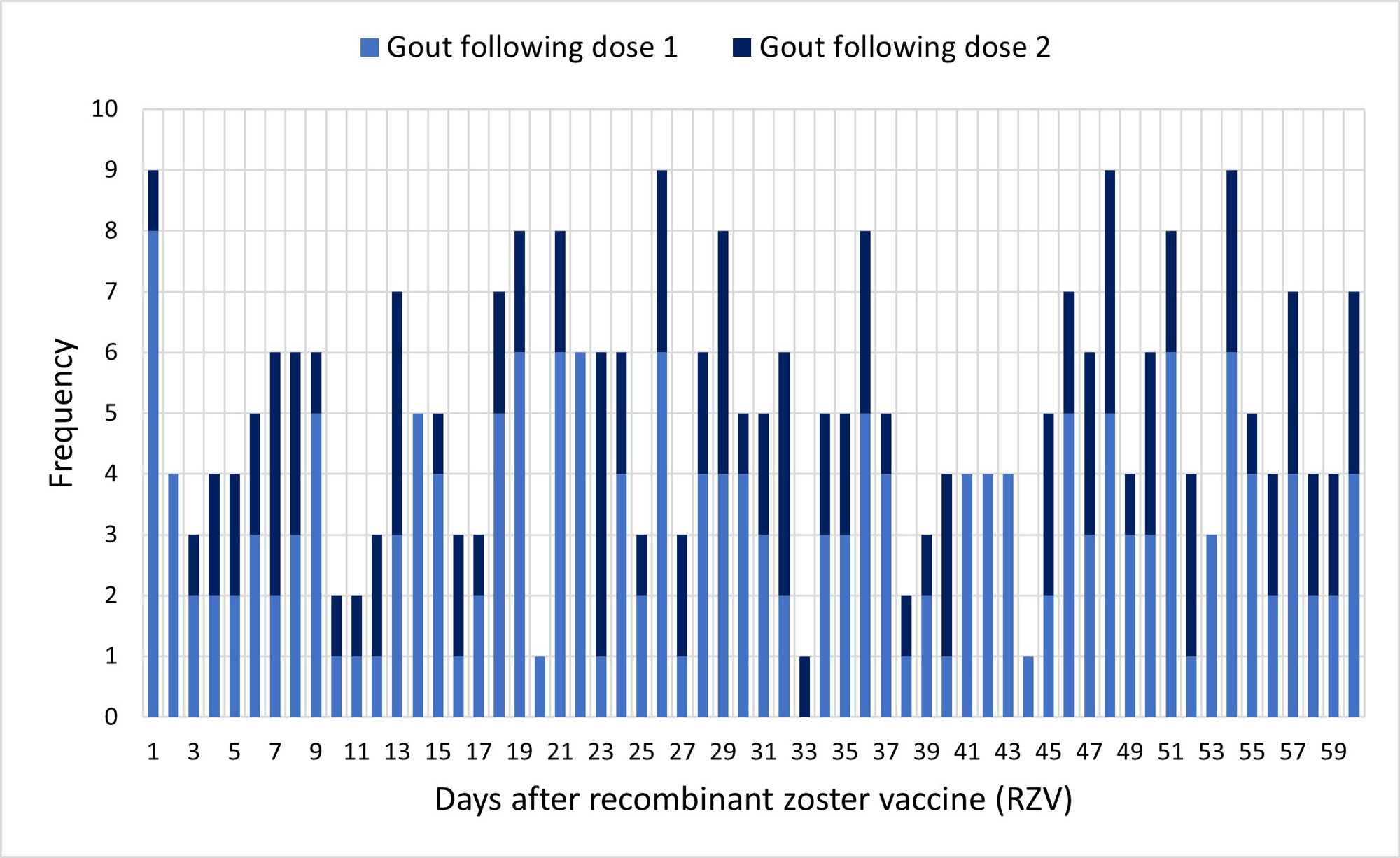
Results: Among 461,323 members with at least one RZV dose, 302 were eligible for the SCRI analysis, with evidence of new-onset gout within 60 days. The analytic cohort had a mean age of 72.5 years (SD = 8.3); 66% (n=199) were male. The most common comorbidities were diabetes (40%; n=120), chronic kidney disease (38%; n=115), and ischemic heart disease (28%; n=83). Figure 2 illustrates the frequency of gout events for each day of follow-up after dose 1 or 2.
In the primary analysis, 153 (50.7%) gout cases occurred during the RW and 149 (49.3%) during the CW with a RR of 1.03 (95% confidence interval [CI] 0.81-1.29). In the secondary analysis of fixed 30-day CWs, the RR was 1.03 (95% CI: 0.81-1.30). All sensitivity analyses had consistent results, with no statistically significant association of RZV with incident gout (Table 1).
Conclusion: This retrospective safety study found no evidence of a statistically significant increased risk of gout in the 30 days following RZV vaccination relative to a 30-day subsequent control window among a large, diverse national patient population of adults ≥50 years of age.
To cite this abstract in AMA style:
Kluberg S, Simon A, Alam S, Peters A, Horgan C, Li D, Moyneur É, Platt R, McMahill-Walraven C, Djibo D, Ma Q, Selvan M, Pernar C, Ziyadeh N, Jamal-Allial A, Daniels K, Spence O, Oraichi D, Seifert H, Franck V, Gamble S, Yun H. Risk of Incident Gout Following Exposure to Recombinant Zoster Vaccine in Adults Aged ≥50 Years in the United States [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/risk-of-incident-gout-following-exposure-to-recombinant-zoster-vaccine-in-adults-aged-%e2%89%a550-years-in-the-united-states/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-incident-gout-following-exposure-to-recombinant-zoster-vaccine-in-adults-aged-%e2%89%a550-years-in-the-united-states/