Session Information
Date: Monday, November 18, 2024
Title: Abstracts: SLE – Treatment II: Non-Cellular Lupus Therapeutics
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Systemic lupus erythematous (SLE) is a systemic inflammatory autoimmune disease which frequently affects the kidneys. Lupus nephritis (LN) is associated with increased morbidity and mortality. Despite immunosuppressive therapies 10-20% of patients will progress to end-stage renal disease (ESRD) within 5 years of diagnosis. Glucagon-like peptide-1 receptor agonists (GLP-1Ra) are a new class of anti-hyperglycemic drugs which have been shown to be beneficial in reducing albuminuria in diabetic patients. This effect is thought to be independent of glycemic control. Studies have suggested that GLP-1Ra may reduce inflammation through multiple pathways and may be beneficial in reducing kidney disease independent of the presence of diabetes. We sought to determine if use of GLP-1Ra in patients with LN is associated with lower risk of progression to ESRD.
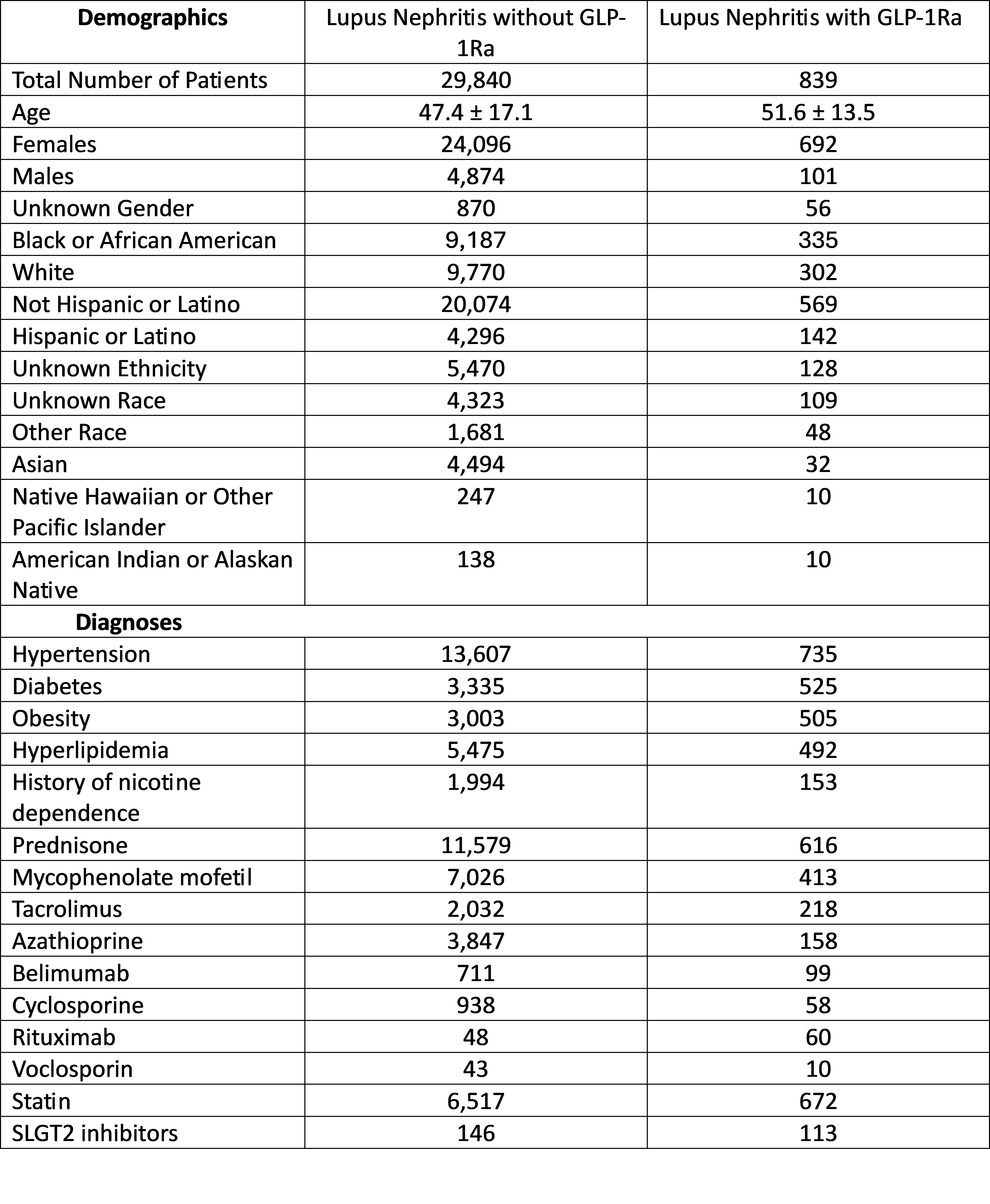
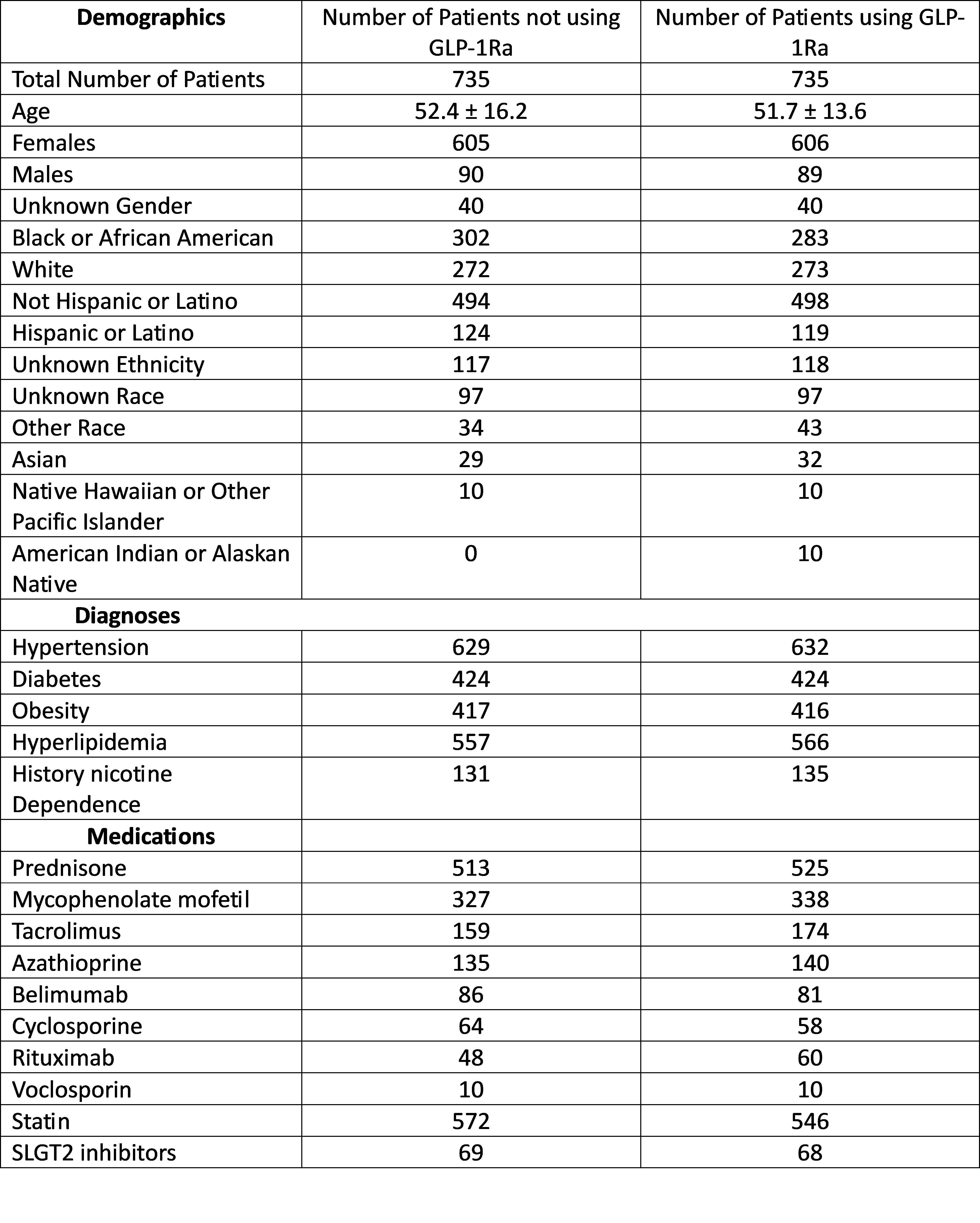
Methods: This is a retrospective, multicenter, observational study using the TrinetX global research database. Using ICD-10 diagnostic codes, patients diagnosed with LN from January 1, 2014 to January 1, 2024, were identified. Patients who were treated with GLP-1Ra any time after LN diagnosis were identified and compared to patients who have never been on GLP-1Ra. Propensity score matching (PSM) was carried out to match age, sex, race, ethnicity, presence of HTN, DM, use of immunosuppressive medications, use of SGLT2 inhibitors, smoking, obesity and statin use. Compare outcome analytic function was utilized to determine risk of ESRD within 5 years among both groups.
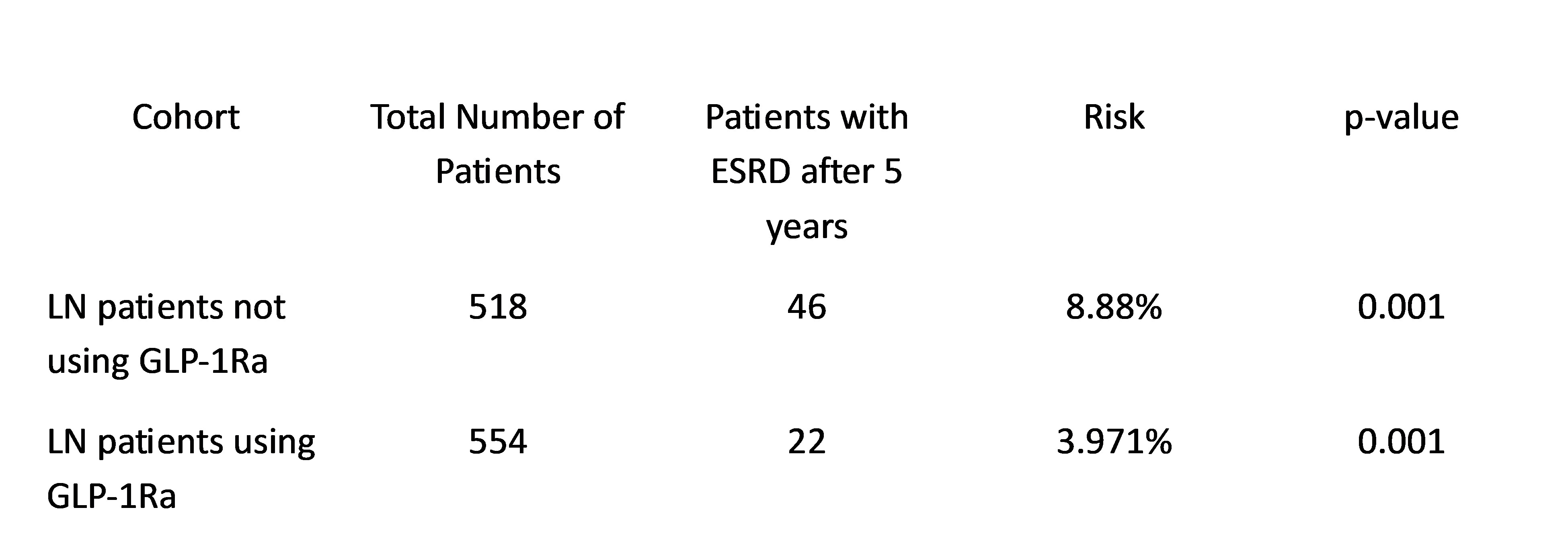
Results: Before matching there were 839 patients with LN taking GLP-1Ra and 29,321 patients with LN who have never been on GLP-1Ra. After matching and excluding patients with ESRD, the number of patients using GLP1Ra was 554 and those who never used GLP-1Ra was 518. After matching, patients who were not on GLP-1Ra were twice as likely to develop end-stage renal disease or dialysis dependence in 5 years after diagnosis compared to patients using GLP-1 agonists (OR 2.35, 95% CI 1.39,3.976).
Conclusion: Among patients with lupus nephritis, GLP-1 receptor agonists may reduce the risk of progression to end-stage renal disease possibly due to benefits in reducing pro-inflammatory mediators.
To cite this abstract in AMA style:
Palmer A, Tan I. Risk of End-Stage Renal Disease Among Patients with Lupus Nephritis on GLP-1 Receptor Agonists: A Retrospective Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/risk-of-end-stage-renal-disease-among-patients-with-lupus-nephritis-on-glp-1-receptor-agonists-a-retrospective-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-end-stage-renal-disease-among-patients-with-lupus-nephritis-on-glp-1-receptor-agonists-a-retrospective-cohort-study/