Session Information
Date: Monday, November 13, 2023
Title: (1264–1307) RA – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Our study aimed to estimate the incidence and risk factors of cancer among rheumatoid arthritis (RA) patients treated with tocilizumab (TCZ) and followed for five years in the French registry (REGATE), which assessed the effectiveness and safety of TCZ.
Methods: We specifically focused on solid cancers, hematological malignancies, and skin cancers including non melanoma skin cancers (NMSC). To collect these data, we developed a pre-defined data collection form and distributed it to participating centers that reported malignancies in REGATE. To identify potential risk factors associated with cancer, we performed a univariate analysis and a multivariate analysis using logistic regression analysis.
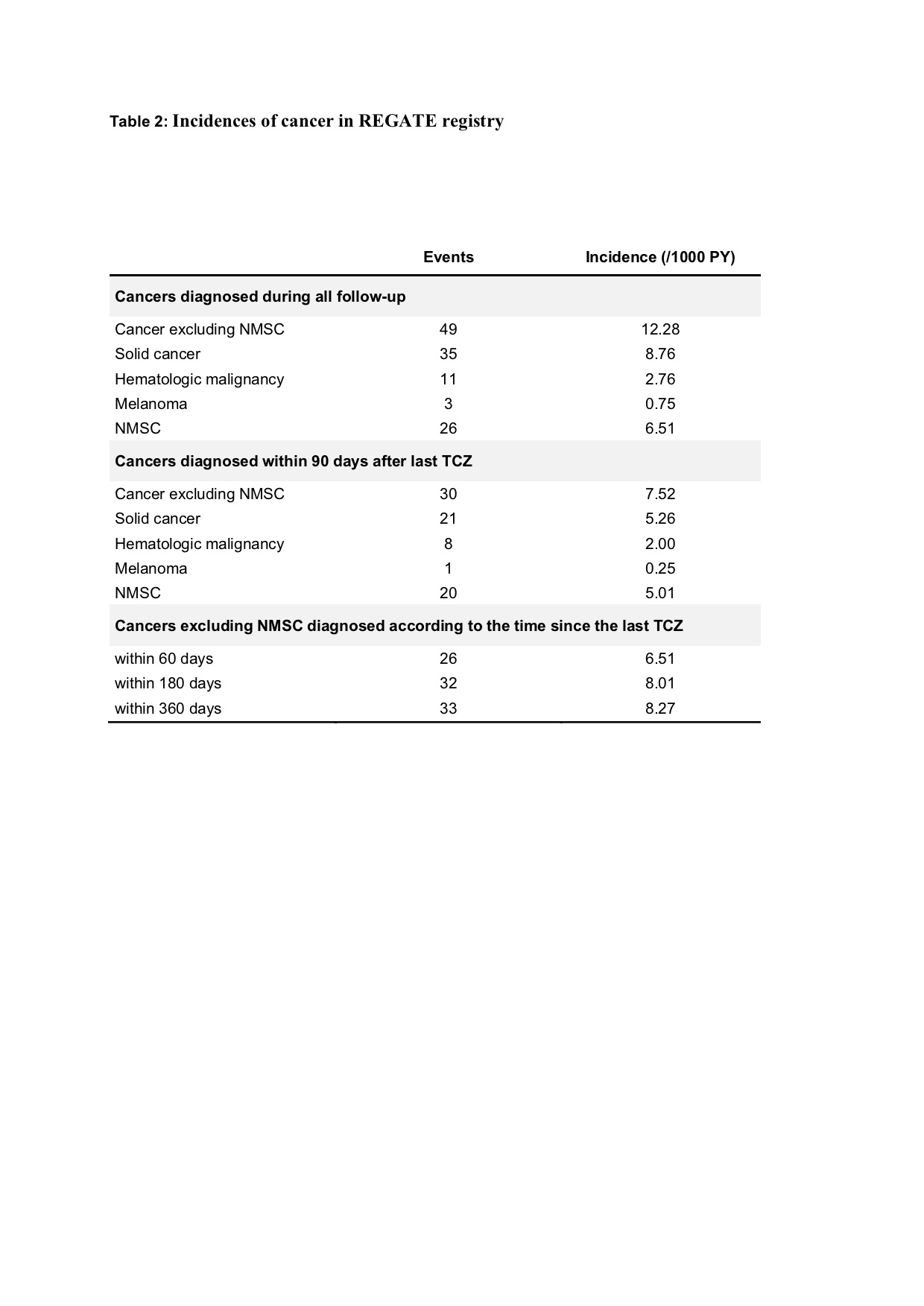
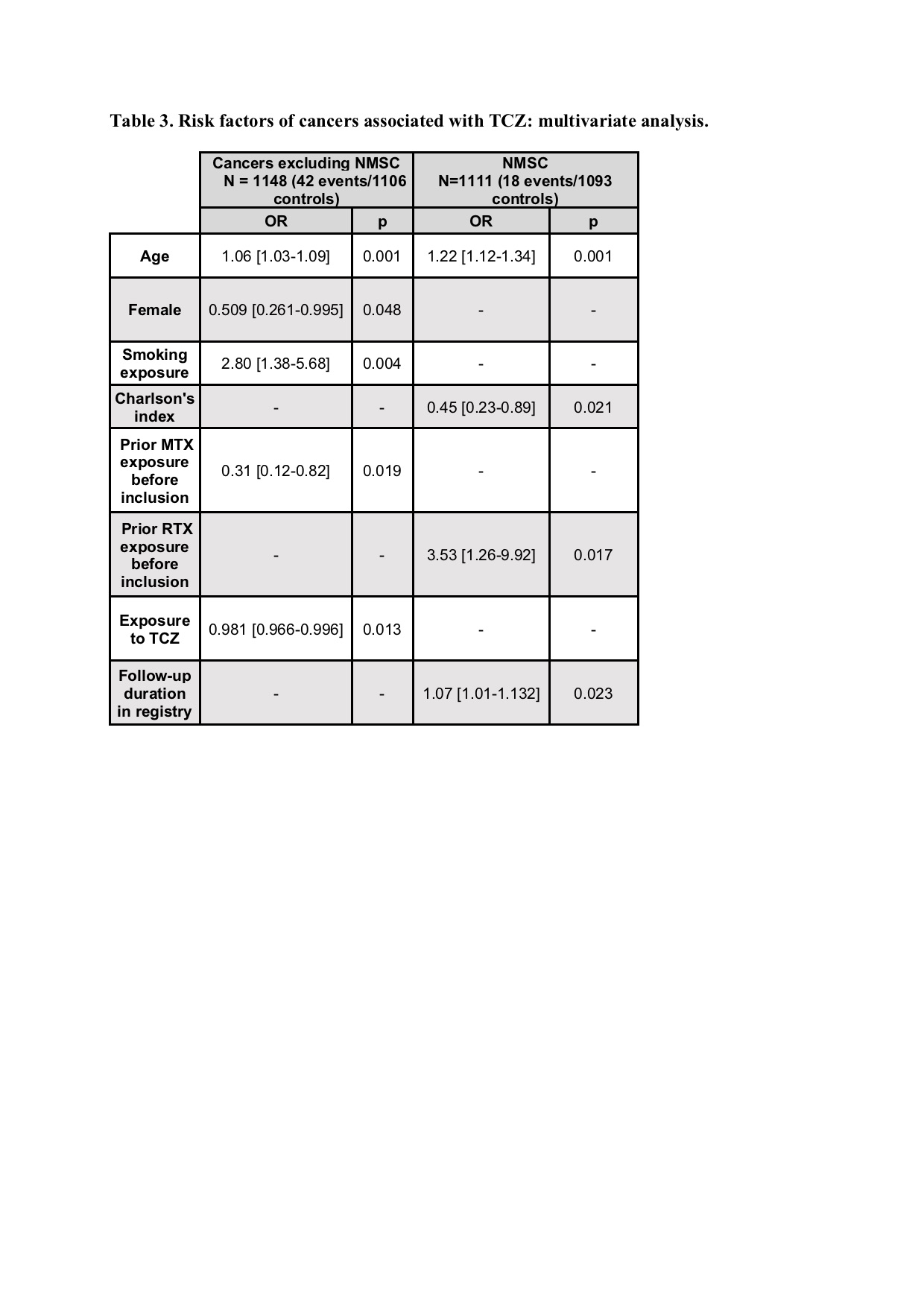
Results: Our study included 1496 patients with RA who were treated with TCZ for a mean duration of 32.0 (±22.0) months and followed for an average of 47 (±15.2) months, resulting in a total exposure of 3990.9 patient-years (PY). Of these patients, 63 (4.2%) were diagnosed with a total of 75 cancers during the follow-up period (35 solid neoplasms, 11 hematological malignancies, 3 melanomas, and 26 NMSC). The overall incidence of cancer excluding NMSC was 8.3 /1000 PY, which was calculated after excluding patients who developed cancer more than 90 days after the last TCZ administration Our multivariate analysis revealed that high age (OR=1.06 [1.03-1.09], p=0.001) and smoking exposure (OR=2.80 [1.38-5.68], p=0.004) were independent risk factors for the group of cancers excluding NMSC.
Conclusion: Our study identified an incidence of cancer comparable to that observed in RA patients treated with other bDMARD and no other risk factor than those known in the general population
To cite this abstract in AMA style:
MOREL J, Wetzman A, Wendling D, Soubrier M, Roth O, Gottenberg J, Goupille P, Mariette X, Lukas C. Risk of Cancer in Patients with Rheumatoid Arthritis Under Tocilizumab: Data from the French National Registry REGATE [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/risk-of-cancer-in-patients-with-rheumatoid-arthritis-under-tocilizumab-data-from-the-french-national-registry-regate/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-cancer-in-patients-with-rheumatoid-arthritis-under-tocilizumab-data-from-the-french-national-registry-regate/