Session Information
Date: Monday, November 9, 2020
Title: Late-Breaking Posters
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Clinician-reported registry data suggest that use of biologics in people with immune-mediated inflammatory diseases (IMIDs) is associated with a lower risk of adverse COVID-19 outcomes compared with no treatment. However, the role of potentially confounding risk-mitigating behaviors is unclear. Given the variation in public health messaging between countries, it is possible that risk mitigating behavior in people with IMIDs will also vary, but this has not been explored to date. We sought to explore risk mitigating behavior using global patient survey data by: (1) determining associations with immunosuppressant treatment type, and (2) characterizing international variation.
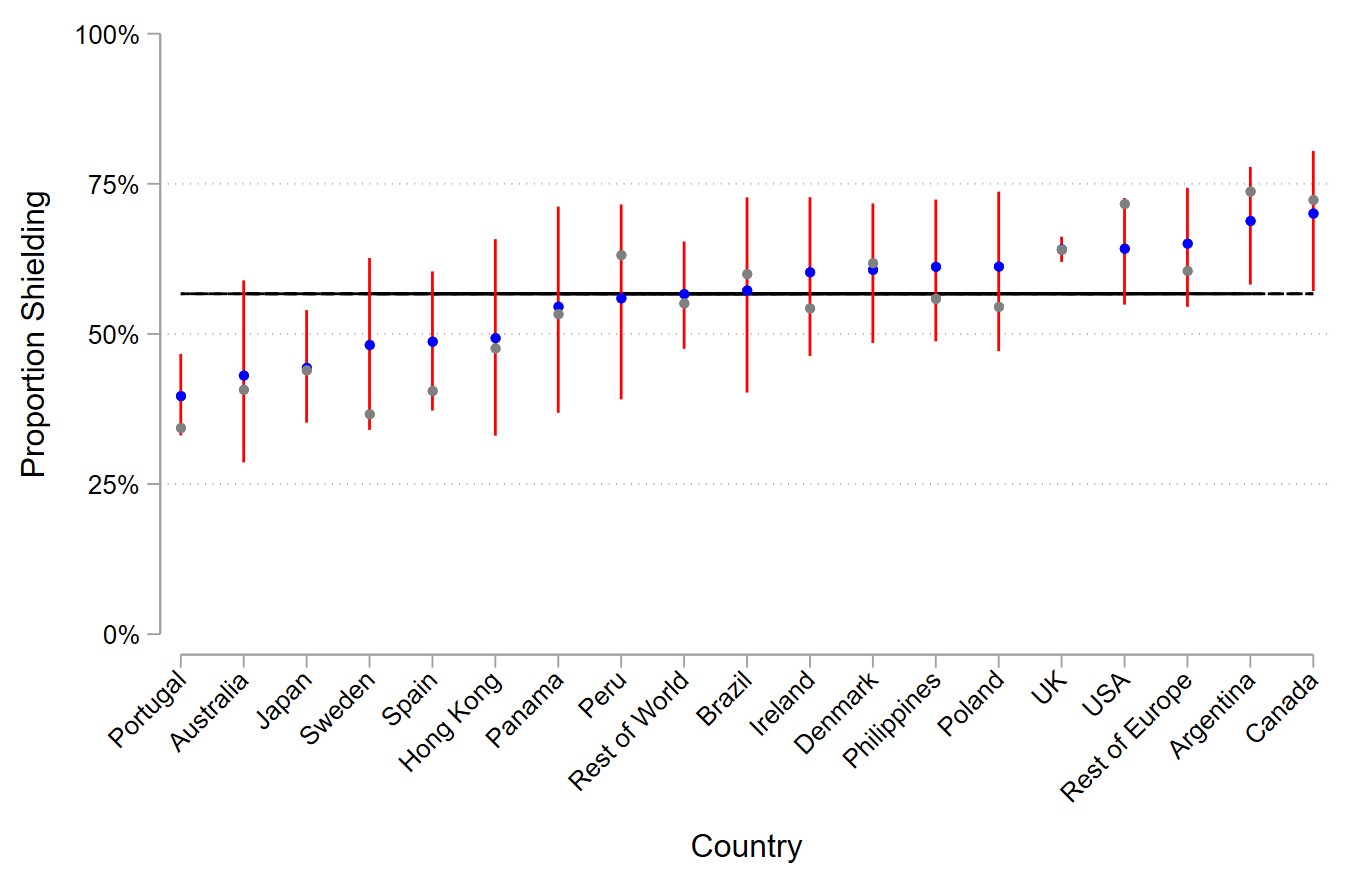
Methods: Online surveys were completed by individuals with rheumatic diseases (UK only) and psoriasis (global) between 20th May and 7th September 2020. Data were collected on diagnosis, treatment types, demographics and risk mitigating behaviors. Multiple logistic regression assessed the association between treatment type and the most stringent risk mitigating behavior (‘shielding’, defined as quarantine/staying home/distancing within the home), adjusting for clinical and demographic characteristics. Incomplete covariates were accounted for using multiple imputation in sensitivity analyses. International variations in shielding were assessed in a mixed effects model, adjusted for clinical and demographic differences between nations and accounting for varying sample sizes. Observed and estimated findings were visualized using a caterpillar plot.
Results: Of 3,714 participants from 74 countries, 2,259 (60.8%) reported the most stringent risk mitigating behavior (i.e. shielding). Use of biologics was associated with higher shielding rates compared to no systemic therapy (odds ratio [OR] 1.65, 95% CI 1.32-2.07) and standard systemic therapy (OR 1.37, 95% CI 1.23-1.52). No differences in shielding was found between standard systemic therapies and no therapy. Shielding was also associated with established risk factors for severe COVID-19 (male sex [OR 1.13, 95% CI 1.02-1.25], obesity [OR 1.46, 95% CI 1.32-1.62], comorbidity burden [OR 1.45, 95% CI 1.21-1.75]), rheumatic disease (OR 1.36, 95% CI 1.26 to 1.47) and a positive screen for anxiety or depression (OR 1.58, 95% CI 1.38-1.80). Findings were unchanged following multiple imputation. After accounting for sample size and clinical/demographic differences, modest differences in the mean proportion of patients shielding were observed across different nations.
Conclusion: Higher rates of shielding among people with IMIDs receiving biologics may contribute to the reported lower risk of adverse COVID-19 outcomes. The observed variation in shielding among treatment groups reinforces the need for clear patient communication on risk mitigation strategies. The findings help inform how clinicians discuss COVID-19 risks with vulnerable patients and will inform public health guidelines as the global COVID-19 pandemic continues to unfold.
To cite this abstract in AMA style:
Yates M, Mahil S, Langan S, De la cruz C, diMeglio P, Dand N, Yiu Z, Mason K, Tsakok T, Meynall F, McAteer H, Weinman J, Gisondi P, Puig Sanz L, Warren R, Capon F, Denis J, Torres T, Griffiths C, Barker J, Hyrich K, Cope A, Bruce I, McInnes I, Sengupta R, Marzo-Ortega H, Brown M, Galloway J, Smith C. Risk Mitigating Behavior in People with Rheumatic Diseases or Psoriasis During the COVID-19 Pandemic Differ by Immunosuppressant Treatment Type: A Patient survey Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/risk-mitigating-behavior-in-people-with-rheumatic-diseases-or-psoriasis-during-the-covid-19-pandemic-differ-by-immunosuppressant-treatment-type-a-patient-survey-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-mitigating-behavior-in-people-with-rheumatic-diseases-or-psoriasis-during-the-covid-19-pandemic-differ-by-immunosuppressant-treatment-type-a-patient-survey-study/