Session Information
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Relapses are common in ANCA-associated vasculitis (AAV), both i) during maintenance treatment after induction of remission, and ii) after immunosuppressive treatment is discontinued. Relapses remain difficult to predict, so patients are often treated with prolonged immunosuppression. This study aimed to determine risk factors for relapse of AAV after successful induction of remission with rituximab.
Methods: This is a post-hoc analysis of the RITAZAREM clinical trial, during which patients 15 years or older with AAV (granulomatosis with polyangiitis or microscopic polyangiitis) and current or historical positive test for anti-proteinase-3 (PR3) or anti-myeloperoxidase (MPO) ANCA who achieved remission after induction with rituximab and glucocorticoids were randomized at month 4 to receive 20 months of rituximab or azathioprine treatment, and were then observed after withdrawal of treatment until relapse (BVAS/WG > 1) or up to 48 months. Rituximab was superior to azathioprine for maintenance of remission. Generalized estimating equations logistic regression was used to identify baseline and time-varying risk factors for relapse by the next visit for the two study phases: maintenance (months 4-24) and off-treatment (months 24-48). Candidate predictors included baseline data (demographics, disease characteristics, ANCA type, treatment assignment), variables during follow-up (laboratory values, patient-reported outcomes), and change compared to Month 4.
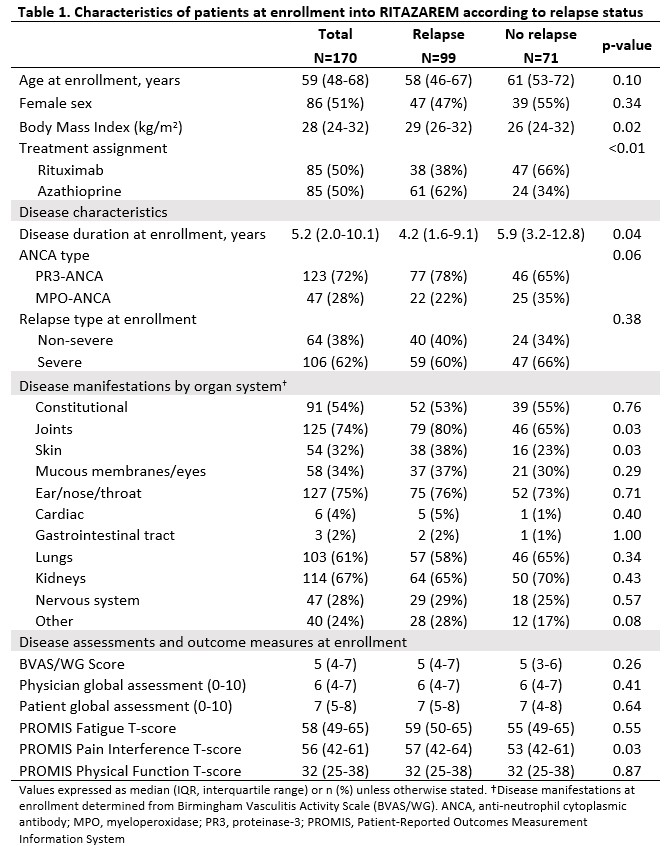
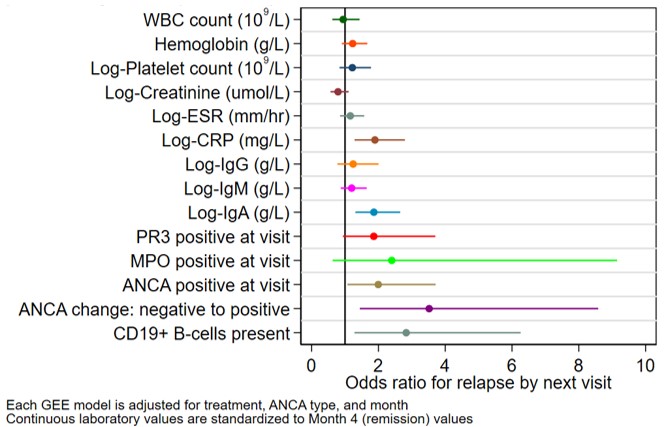
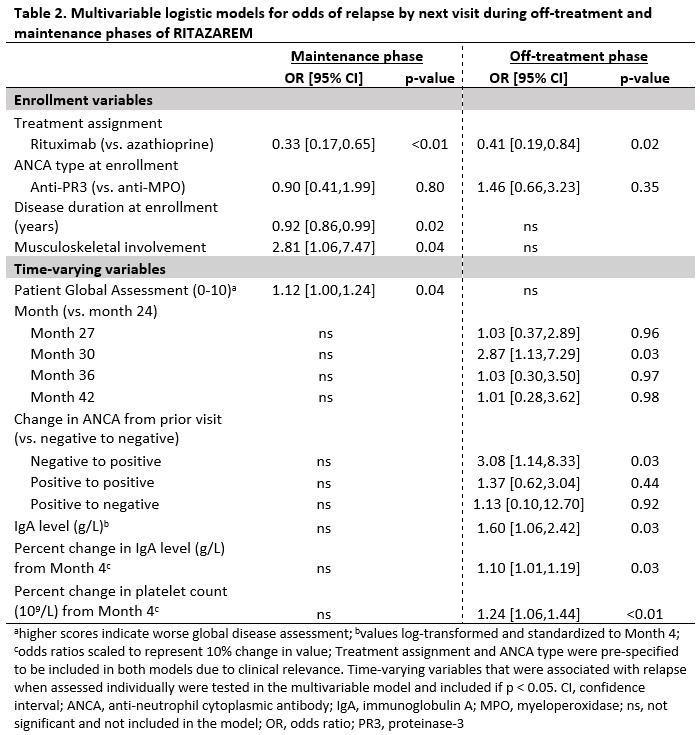
Results: 170 patients with a median (IQR) age of 59 (48-68) years and disease duration of 5 (2-10) years were randomized to rituximab or azathioprine (Table 1). There were 99 relapses, 46 during the maintenance phase. During the maintenance phase, joint involvement (odds ratio (OR) [95% confidence interval (CI)]: 2.8 [1.1, 7.5], p=0.04) and higher patient global assessment score (range 0-10) (OR [95% CI]: 1.1 [1.0, 1.2], p=0.04) associated with higher odds for relapse. Longer disease duration associated with lower odds for relapse (OR [95% CI]: 0.90 [0.86, 0.99], p=0.02), (Table 2). During the off-treatment phase, presence of CD19+ B-cells (≥ 0.01 cells/109/L or ≥1 %) associated with 2.8-fold increased odds of relapse by next visit (OR [95% CI]: 2.8 [1.3, 6.3], p=0.01), and change in ANCA from negative to positive associated with 3.5-fold increased odds of relapse (OR [95% CI]: 3.5 [1.4, 8.6], p=0.006) when assessed individually (Figure 1). After stratification by treatment, the association with presence of CD19+ B-cells and change in ANCA was observed for the rituximab but not the azathioprine group. In a multivariable model, the change in ANCA to positive and markers of immune reconstitution (change in platelets and immunoglobulins) were associated with relapse by next visit (Table 2).
Conclusion: Risk factors for relapse in AAV differ according to treatment phase. Laboratory values anticipate subsequent relapses during the off-treatment phase, including markers of inflammation and immune reconstitution. Return of ANCA and B-cells is informative. These data support close monitoring of laboratory values at regular intervals to identify patients at risk for relapse after withdrawal of treatment.
Adjusted odds ratios for relapse by next visit for follow-up laboratory variables during the off-treatment phase (months 24-48). Each variable is included separately in a logistic GEE model, adjusting for treatment (rituximab vs. azathioprine), ANCA type, and month. Continuous laboratory values are standardized to Month 4 values (remission) to allow comparison of effect sizes. Presence of CD19+ B-cells is defined as ≥0.01 cells per 109/L or ≥1 percent. ANCA, anti-neutrophil cytoplasmic antibody; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Ig, immunoglobulin; GEE, generalized estimating equations; MPO, myeloperoxidase; PR3, proteinase_3; WBC, white blood cell.
To cite this abstract in AMA style:
Romich E, Baker J, Green I, Rhee R, McAlear C, Specks U, Smith R, Jayne D, Merkel P. Risk Factors for Relapse in ANCA-Associated Vasculitis Among Patients with Relapse After Induction of Remission with Rituximab [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/risk-factors-for-relapse-in-anca-associated-vasculitis-among-patients-with-relapse-after-induction-of-remission-with-rituximab/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-relapse-in-anca-associated-vasculitis-among-patients-with-relapse-after-induction-of-remission-with-rituximab/