Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) is one of the main pulmonary complications of systemic sclerosis (SSc), with a prevalence of 50-60%. SSc-ILD progression can occur in 25-30% of patients and has high morbidity and mortality. Identifying prognostic markers of SSc-ILD progression early in disease course is therefore of great clinical value. Prior studies have identified African American race, male sex, presence of anti-topoisomerase I antibody, and the diffuse cutaneous(dc) subtype as risk factors for ILD progression. These studies have often included patients with both limited and diffuse cutaneous SSc or have included clinical trial cohorts with unclear generalizability to patients receiving standard management. In this study, we investigated a real-world cohort of patients with early dcSSc enrolled in the Prospective Study of Early Systemic Sclerosis (PRESS) to identify risk factors for SSc-ILD progression.
Methods: PRESS is a multicenter prospective cohort study of adults with early dcSSc, defined as a disease duration ≤ 2 years from the first non-Raynaud symptom. All participants met 2013 ACR/EULAR Classification Criteria for SSc and were recruited from 12 expert Scleroderma Centers in the U.S. from April 2012 to July 2021. Subjects were included if they had SSc-ILD based on a baseline chest CT scan, baseline pulmonary function tests (PFTs), and follow-up PFTs within 12 (+3) months. SSc-ILD progression was defined as ≥ 5% relative decline in forced vital capacity (FVC) within 12 (+3) months. We used the Student’s t test, Wilcoxon rank sum test, chi square test, and Fisher’s exact test, as appropriate, to compare baseline characteristics between participants who did and did not have SSc-ILD progression. We performed univariate logistic regression followed by stepwise selection of variables (p-value for entry = 0.30, p-value for remaining = 0.35) and multivariable logistic regression to determine the patient characteristics associated with SSc-ILD progression. We used multiple imputation (20 imputed sets) to handle missing data for logistic regression. Statistical significance in the univariate and multivariable models was defined as a p-value < 0.05.
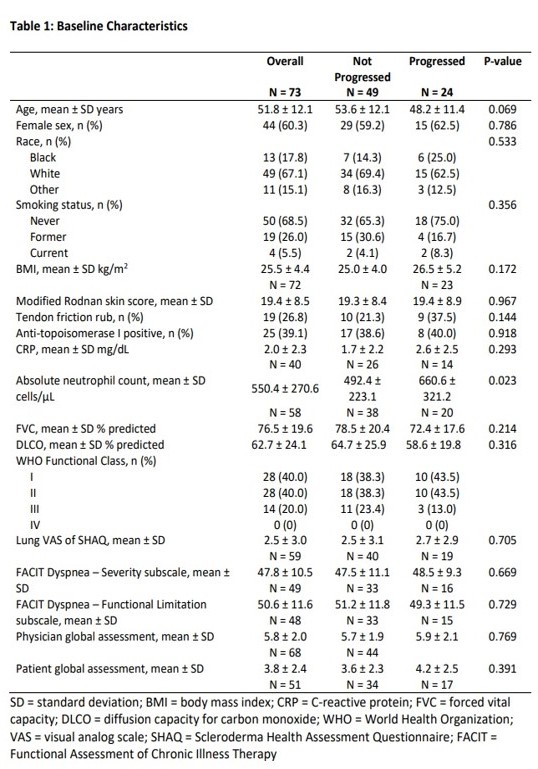
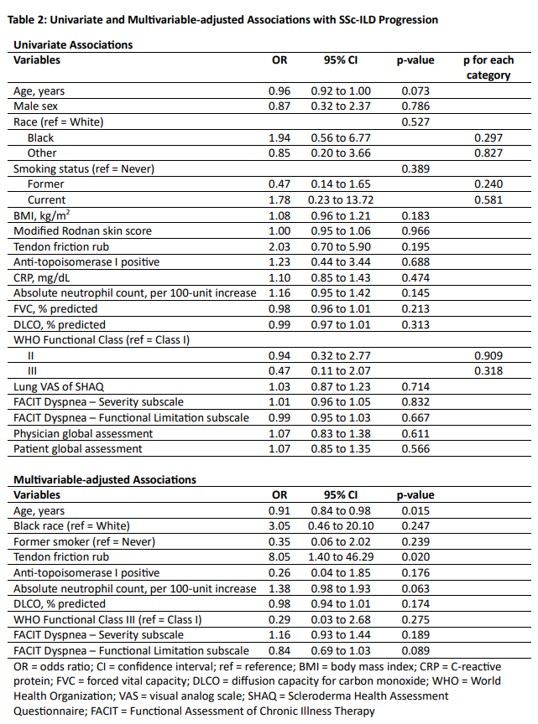
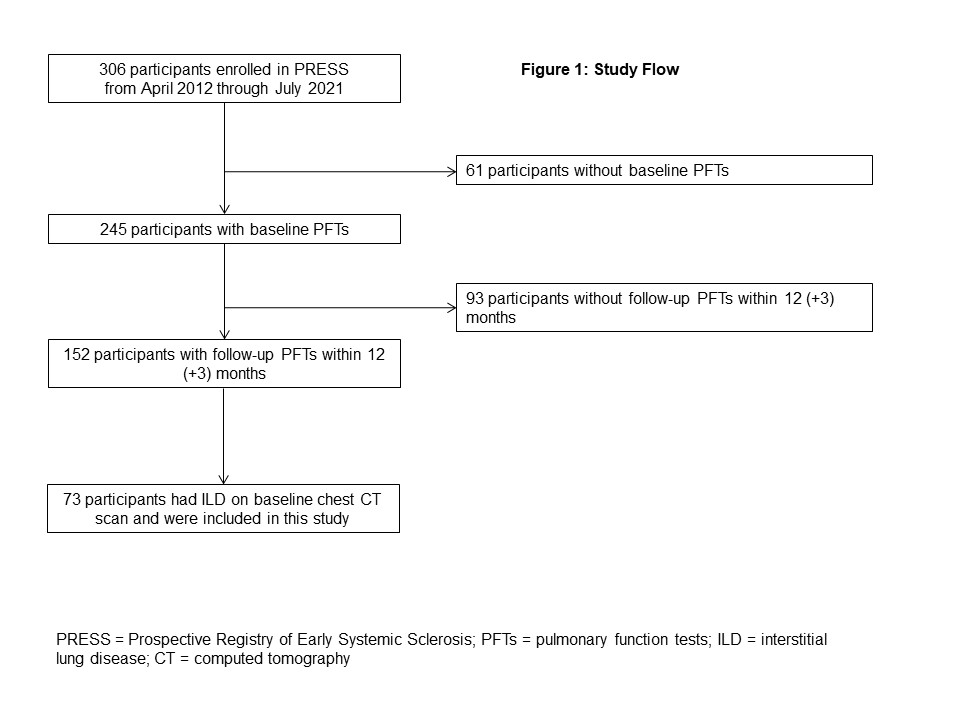
Results: Seventy-three patients with dcSSc-ILD were included in this study (Figure 1). The mean (standard deviation, SD) age was 51.8 (12.1) years; 60.3% were female (Table 1). The mean (SD) baseline FVC% was 76.5 (19.6)%. Thirty-three percent had progression of SSc-ILD. In our multivariable-adjusted model, younger age (odds ratio [OR] 0.91, 95% confidence interval [CI] 0.84 to 0.98, p=0.0148) and the presence of tendon friction rubs (TFR) (OR 8.05, 95% CI 1.40 to 46.29, p=0.0195; Table 2) were statistically significantly associated with ILD progression.
Conclusion: In a cohort of patients with early dcSSc, younger age and TFRs were associated with SSc-ILD progression. Including patients with early dcSSc and TFRs in SSc-ILD clinical trials may be a viable strategy to enrich for progressors.
To cite this abstract in AMA style:
Urgiles J, Huang S, Assassi S, Castelino F, Domsic R, Frech T, Gordon J, Hant F, Hinchcliff M, Shah A, Shanmugam V, Steen V, Khanna D, Bernstein E. Risk Factors for Progression of Interstitial Lung Disease in Patients with Early Diffuse Cutaneous Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/risk-factors-for-progression-of-interstitial-lung-disease-in-patients-with-early-diffuse-cutaneous-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-progression-of-interstitial-lung-disease-in-patients-with-early-diffuse-cutaneous-systemic-sclerosis/