Session Information
Date: Sunday, November 7, 2021
Title: Abstracts: Epidemiology & Public Health I: Risk in Rheumatic Diseases (0988–0991)
Session Type: Abstract Session
Session Time: 4:15PM-4:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is a key treatment for patients with lupus and other rheumatic diseases; however, the known risk factors for HCQ retinopathy (its major toxicity), are mostly derived from prevalent case analyses. We determined the risk factors for incident HCQ retinopathy within a large-scale, longitudinal incident user cohort.
Methods: We conducted a nested case-control study within a large cohort of incident HCQ users identified from a US integrated health network who continued HCQ for ≥5 years during the study period, 1997-2020. We assessed the outcome of retinopathy by prospective assessment of spectral domain-optical coherence tomography (SD-OCT) scans. All scans were reviewed by an expert ophthalmologist (RM), and a second expert ophthalmologist (MM) reviewed all abnormal scans and a random subset of normal scans. Discrepant cases were adjudicated by consensus between the two readers. We identified the dates of earliest abnormal scans. Each SD-OCT scan was graded as mild, moderate, or severe retinopathy, no retinopathy, or non-HCQ retinopathy (such as macular degeneration), and retinopathy cases were subclassed as parafoveal or pericentral pattern. Cases with incident HCQ retinopathy were matched with up to 5 controls by age, sex, and HCQ initiation year using risk set sampling. Using pharmacy dispensing records, we assessed HCQ duration of use, cumulative dose, average daily dose, and dose per actual body weight (ABW) and ideal body weight (IBW). Candidate risk factors also included race/ethnicity and chronic kidney disease (CKD). We used conditional logistic regression to assess the association between potential risk factors and the risk of HCQ retinopathy overall, by severity, and by pattern.
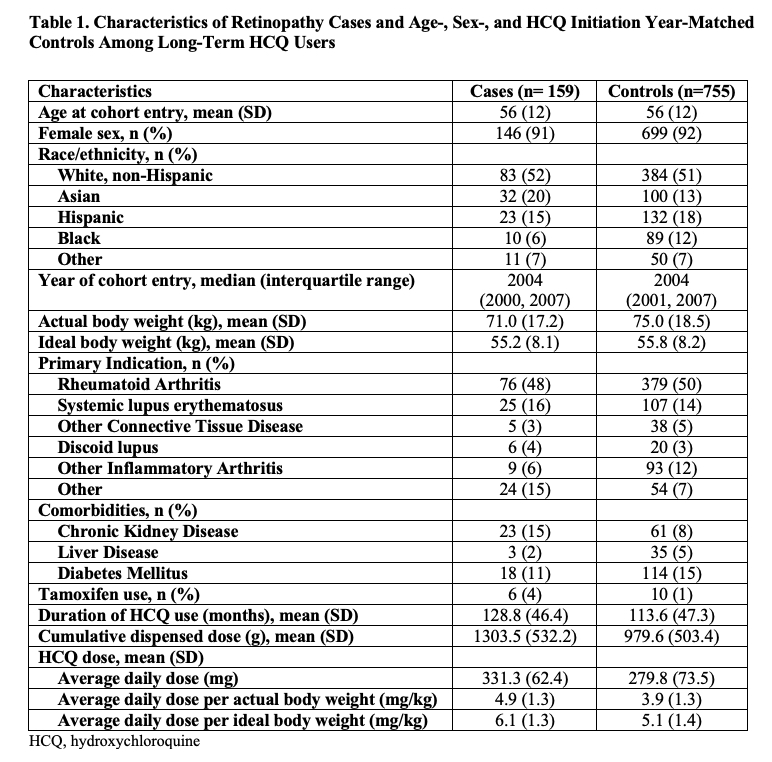
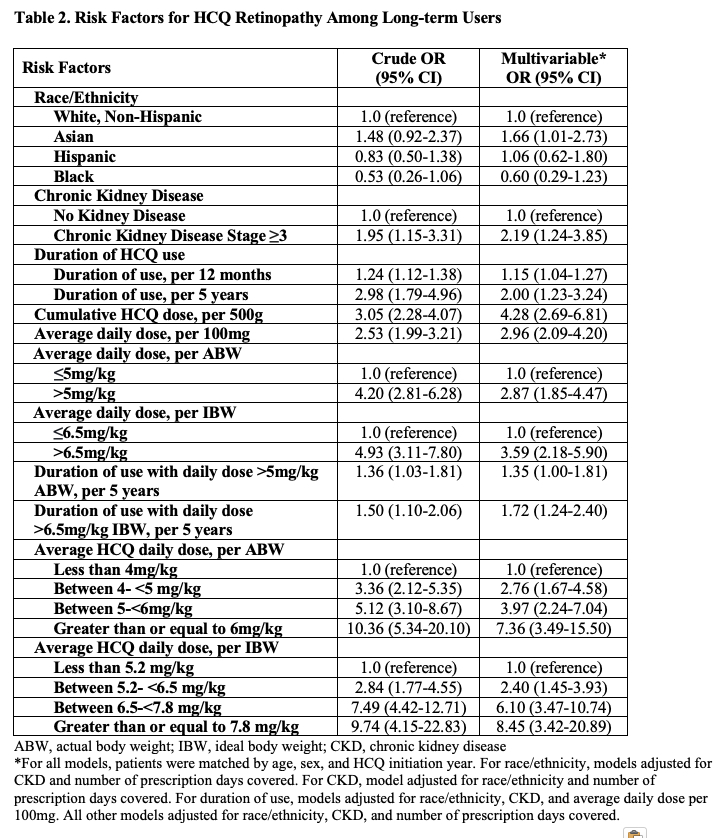
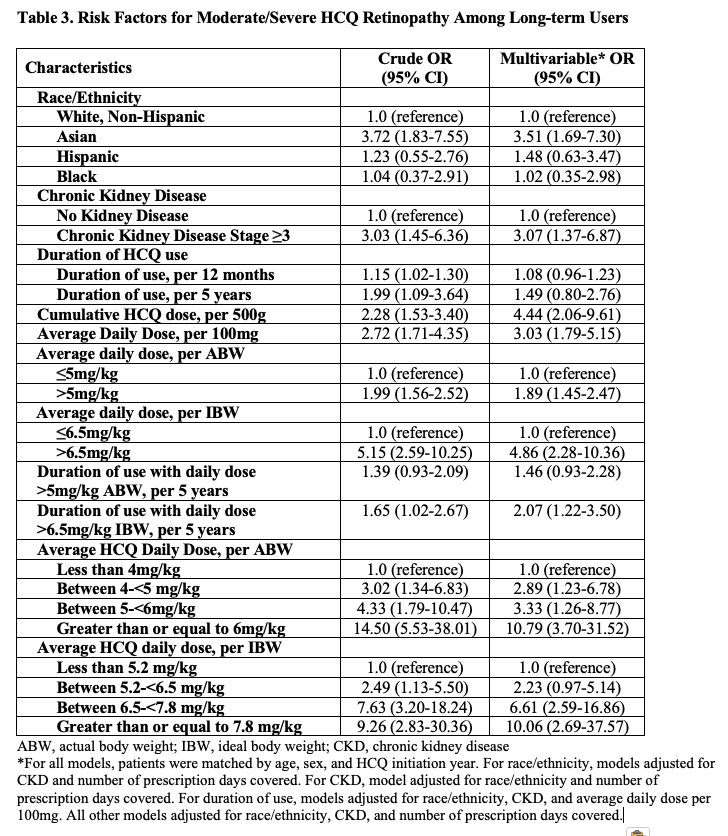
Results: Of 4,899 long-term HCQ users, we identified 164 patients with incident HCQ retinopathy (100 mild, 38 moderate, and 26 severe cases), with a parafoveal pattern in 131 and pericentral pattern in 33 patients. We matched 159 cases with 755 controls. The mean age at HCQ initiation was 56 years; over 90% were female (Table 1). The risk of HCQ retinopathy doubled for every additional 5 years of use (Table 2). Cumulative dose was associated with an adjusted risk ratio of 4.28 (95% CI 2.69-6.81) per additional 500g, and dosing category per mg/kg of ABW or IBW strongly correlated with the risk of retinopathy. Asian patients had an increased risk of HCQ retinopathy overall, moderate/severe grade, and the pericentral pattern. CKD was associated with 2x higher risk of overall retinopathy and a 3x higher risk of moderate/severe retinopathy (Table 3).
Conclusion: The risk of incident HCQ retinopathy increases with higher dosing per ABW and per IBW in a dose-response manner. We found cumulative HCQ dose, duration of use, CKD, and Asian race to be independent risk factors for incident HCQ retinopathy including moderate/severe cases. Patients with these risk factors may warrant closer monitoring.
To cite this abstract in AMA style:
Jorge A, Melles R, Conell C, Lu N, Marmor M, Young L, McCormick N, Zhang Y, Choi H. Risk Factors for Hydroxychloroquine Retinopathy and Its Subtypes – Prospective Adjudication Analysis of 4,899 Incident Users [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/risk-factors-for-hydroxychloroquine-retinopathy-and-its-subtypes-prospective-adjudication-analysis-of-4899-incident-users/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-hydroxychloroquine-retinopathy-and-its-subtypes-prospective-adjudication-analysis-of-4899-incident-users/